Mandestrobin, A Novel and Safty Fungicide

Figure 1 The structure of Mandestrobin
Identity, physical/chemical/technical properties and methods of analysis
The conclusions drawn in this report were based on the following guidance documents: SANCO/3030/99 rev.4 (European Commission, 2000), SANCO /10597/2003 – rev. 10.1 (European Commission, 2012), and SANCO/825/00 rev. 8.1 (European Commission, 2010). The active substance, Mandestrobin, has a minimum purity of 940 g/kg as manufactured. While the impurities, except for xylenes, are not known to be relevant (refer to Section 2). Appendix A provides the key information regarding the identity of Mandestrobin, including its physical and chemical properties. Regarding solvent solubility, two studies were conducted, yielding significantly different results, thus creating a data gap that necessitates an additional study. An HPLC-UV method can be used to analyze the active substance in both the technical material and formulation. The residue definition for all matrices is Mandestrobin, and the DFG S19 method is validated for oily matrices, while the QuEChERS method is validated for dry, acidic, and high water content matrices. Since no maximum residue limits (MRLs) are proposed for products of animal origin, an analysis method is not required for them. LC-MS/MS methods are available for analyzing soil, water, and air samples. In terms of health hazards, the active substance, Mandestrobin, is not classified under Regulation (EC) No 1272/2008 (CLP Regulation), eliminating the need for an analysis method for body fluids and tissues.Mammalian toxicity
The toxicological profile of Mandestrobin, assessed by experts, demonstrated its low toxicity across various studies. The compound consists of two isomers, R and S, with extensive absorption, distribution, metabolism, and excretion observed. Mandestrobin showed minimal acute toxicity when administered orally, dermally, or via inhalation, with no skin sensitization observed. In repeat dose toxicity studies, the liver was identified as the target organ, but no significant adverse effects were observed. The highest dose tested in a 1-year dog study resulted in a short-term no observed adverse effect level (NOAEL) of 19 mg/kg bw per day. No adverse effects were observed in dermal and immunotoxicity studies, up to the highest tested doses. Genotoxicity tests indicated no genotoxic potential for Mandestrobin. In a long-term rat study, a systemic toxicity NOAEL of 26.7 mg/kg bw per day was established, with increased sex-cord stromal tumors in ovaries observed at high doses. However, these tumors were determined to be unrelated to treatment due to various factors. The multigeneration rat study showed no adverse effects on reproductive parameters, while developmental toxicity studies indicated a developmental NOAEL of 300 mg/kg bw per day in rats, with limited findings of malformations. Neurotoxicity studies demonstrated a decrease in locomotor activity, leading to an acute neurotoxic NOAEL of 1000 mg/kg bw.
Environmental fate and behaviour
The toxicological assessment of Mandestrobin, conducted during the Pesticides Peer Review Meeting in November 2014, evaluated its environmental fate and behavior. Mandestrobin, a racemic mixture of R-isomer and S-isomer, underwent separate studies to determine degradation rates in soil and water. No isomerization occurred during these studies, and no studies with the racemic mixture were available. The environmental fate section presented results as a combination of data from both isomers, unless specified. The regulatory dossier lacked information on the environmental behavior of individual isomers of metabolites 2-COOH-S-2200 and 5-COOH-S-2200, which contain chiral carbon atoms. The degradation rates or interconversion between isomers remain unknown. However, considering the large margins of safety in the risk assessments, the uncertainty regarding the toxicity and contribution of isomers to total residue levels of these metabolites did not affect the conclusion of low terrestrial and aquatic risk for the evaluated uses. In soil incubation studies under aerobic conditions, Mandestrobin exhibited moderate to high persistence, with the major metabolite 5-COOH-S-2200 showing similar persistence. Metabolite 2-COOH-S-2200, detected at specific sampling points, triggered a groundwater exposure assessment due to its presence. Both metabolites exhibited moderate to high persistence. Mandestrobin showed medium to low mobility in soil, while the metabolites exhibited very high to low mobility. Soil degradation rates of Mandestrobin were found to be pH-dependent, with differential degradation rates observed under acidic and neutral/alkaline soils.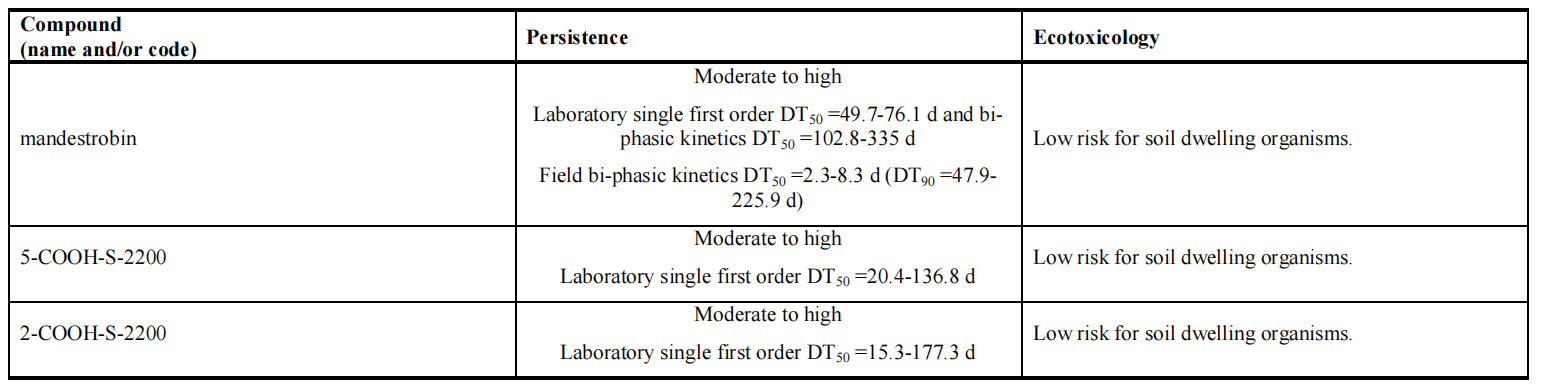
Applications
Mandestrobin is a fungicidal active substance that finds application in the field of agriculture for the control of various plant diseases. It belongs to the strobilurin class of fungicides, which are known for their broad-spectrum activity and systemic properties. Mandestrobin acts by inhibiting the mitochondrial respiration of fungi, leading to the suppression of fungal growth and disease development. The compound has been extensively used in crop protection programs worldwide. One of the major applications of Mandestrobin is in the protection of cereal crops. It has proven effective against a range of fungal pathogens that commonly affect cereals, including powdery mildew, leaf rust, and Septoria leaf blotch. By preventing the spread and development of these diseases, Mandestrobin helps to maintain healthy plant growth and maximize crop yields. In addition to cereals, Mandestrobin is also utilized in the protection of other important crops such as grapes, vegetables, and fruit trees. It has demonstrated efficacy against diseases like downy mildew, gray mold, and apple scab, among others. By providing effective control against these pathogens, Mandestrobin contributes to the overall health and quality of the crops, ensuring better yields and higher market value. The application of Mandestrobin can be done through foliar sprays or seed treatments, depending on the specific crop and disease management requirements. The compound is formulated into different formulations such as suspensions, emulsifiable concentrates, or wettable powders to facilitate its application and ensure even distribution on plant surfaces. Manufacturers provide guidelines on proper dosage rates and timing of application to optimize its effectiveness while minimizing any potential negative impacts. One of the key advantages of Mandestrobin is its favorable environmental profile. It exhibits low toxicity to mammals, birds, and aquatic organisms when used according to recommended practices. Furthermore, its degradation products do not pose significant risks to the environment. These attributes make Mandestrobin a valuable tool for integrated pest management strategies, enabling farmers to protect their crops effectively while minimizing potential harm to non-target organisms and the surrounding ecosystem. As with any pesticide, it is important to follow label instructions and use Mandestrobin responsibly. Applicators should be trained in proper handling, storage, and disposal practices to ensure safety and prevent any adverse effects on human health or the environment. Additionally, adherence to resistance management strategies, including the rotation of different fungicides with distinct modes of action, is crucial to mitigate the development of resistant fungal strains.Summary
Mandestrobin plays a significant role in modern agriculture by providing effective control of fungal diseases in various crops. Its broad-spectrum activity, systemic properties, and favorable environmental profile make it a valuable tool for sustainable disease management. When used responsibly and in accordance with recommended practices, Mandestrobin contributes to the production of healthy crops, higher yields, and overall food security.Mandestrobin, a new active substance, underwent the approval process according to Regulation (EC) No 1107/2009. The application was submitted by Sumitomo Agro Europe S.A.S. on 18 December 2012, and the rapporteur Member State (RMS) Austria deemed it admissible on 31 January 2013. The RMS provided the initial evaluation in the Draft Assessment Report (DAR), which was received by EFSA on 31 January 2014. Following consultation on the DAR, it was determined that additional information and expert consultation in various areas, including mammalian toxicology and environmental fate, were required. In accordance with Article 12 of the Regulation, EFSA was tasked with adopting a conclusion on whether Mandestrobin meets the approval criteria specified in Article 4 of the Regulation. The conclusion also addresses the assessment required under Regulation (EC) No 396/2005, assuming that the active substance is approved without restrictions affecting residue assessment. The conclusions provided in the report were based on the evaluation of the representative use of Mandestrobin as a fungicide on oilseed rape. Overall, the evaluation highlighted the need for additional information and further assessment in specific areas, but also provided insights into the efficacy and potential risks associated with the use of Mandestrobin in agricultural settings. The conclusions of the report emphasized the importance of addressing the identified data gaps and potential concerns to ensure the safe and effective use of Mandestrobin.Reference
- ACD/ChemSketch, Advanced Chemistry Development, Inc., ACD/Labs Release: 12.00 Product version: 12.00 (Build 29305, 25 Nov 2008)
- Austria, 2014. Draft Assessment Report (DAR) on the active substance mandestrobin prepared by the rapporteur Member State Austria in the framework of Regulation (EU) No 1107/2009, January 2014. Available online: www.efsa.europa.eu
- Austria, 2015. Revised Assessment Report on mandestrobin, compiled by EFSA, March 2015. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2007. Opinion on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil. The EFSA Journal 2007, 622, 1–32, doi:10.2903/j.efsa.2008.622
- EFSA (European Food Safety Authority), 2015. Peer Review Report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance mandestrobin. Available online: www.efsa.europa.eu
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2012. Guidance on Dermal Absorption. EFSA Journal 2012;10(4):2665, 30 pp. doi:10.2903/j.efsa.2012.2665
- European Commission, 1999. Guidelines for the generation of data concerning residues as provided in Annex II part A, section 6 and Annex III, part A, section 8 of Directive 91/414/EEC concerning the placing of plant protection products on the market, 1607/VI/97 rev.2, 10 June 1999.
- European Commission, 2000. Technical Material and Preparations: Guidance for generating and reporting methods of analysis in support of pre- and post-registration data requirements for Annex II (part A, Section 4) and Annex III (part A, Section 5) of Directive 91/414. SANCO/3030/99 rev.4, 11 July 2000.
- European Commission, 2002a. Guidance Document on Terrestrial Ecotoxicology under Council Directive 91/414/EEC. SANCO/10329/2002 rev.2 final, 17 October 2002.
- European Commission, 2002b. Guidance Document on Aquatic Ecotoxicology under Council Directive 91/414/EEC. SANCO/3268/2001 rev 4 (final), 17 October 2002.
- European Commission, 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000-rev. 10 – final, 25 February 2003.
- European Commission, 2004. Guidance Document on Dermal Absorption. SANCO/222/2000 rev. 7, 19 March 2004.
- European Commission, 2010. Guidance Document on residue analytical methods. SANCO/825/00 rev. 8.1, 16 November 2010.
- European Commission, 2011. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. SANCO 7525/VI/95 – rev.9. March 2011. pp.1–46.
- European Commission, 2012. Guidance Document on the Assessment of the Equivalence of Technical Materials of Substances Regulated under Council Directive 91/414/EEC. SANCO/10597/2003 –rev. 10.1, 13 July 2012.