Baloxavir Marboxil and Influenza
Abstract
Influenza is an acute respiratory infectious disease caused by the influenza virus, which is mainly transmitted through droplets in the air, contact between susceptible people and infected people, or contact with contaminated items. It is usually a seasonal disease that breaks out once a year, killing thousands of people worldwide. Although rare, the mutation of the virus may cause human infection and spread rapidly, further leading to a pandemic (an infection that spreads worldwide) and causing millions of deaths.
What is influenza?
Influenza is an acute respiratory infectious disease caused by the influenza virus, which is mainly transmitted through droplets in the air, contact between susceptible people and infected people, or contact with contaminated items. It is usually a seasonal disease that breaks out once a year, killing thousands of people worldwide. Although rare, the mutation of the virus may cause human infection and spread rapidly, further leading to a pandemic (an infection that spreads worldwide) and causing millions of deaths.
Symptoms of flu include sudden fever, cough, sneezing, runny nose and severe discomfort, although it can also include vomiting, diarrhea and nausea. Influenza has plagued humans for hundreds of years. Because of its highly variable nature, it may last for hundreds of years. With the increasing awareness of the influenza virus, people pay more and more attention to the prevention and treatment of influenza.
How to treat the flu?
In addition to vaccine prevention, treatment with antiviral drugs is an important component of current intervention strategies to prevent progression and reduce morbidity and mortality in the global response to influenza. Currently developed antiviral drugs mainly by hindering the process of virus proliferation, thereby effectively inhibiting the spread of the virus. There are three main types of antiviral drugs commonly used in the treatment of influenza: M2 ion channel inhibitors represented by Amantadine, neuraminidase inhibitors represented by Zanamivir, and polymerase inhibitors such as Balofloxacin and Fapiravir.
Introduction
Baloxavirmarboxil (trade name: Xofluza) is a new anti-influenza drug developed by Shionogi of Japan for influenza A and B viruses.
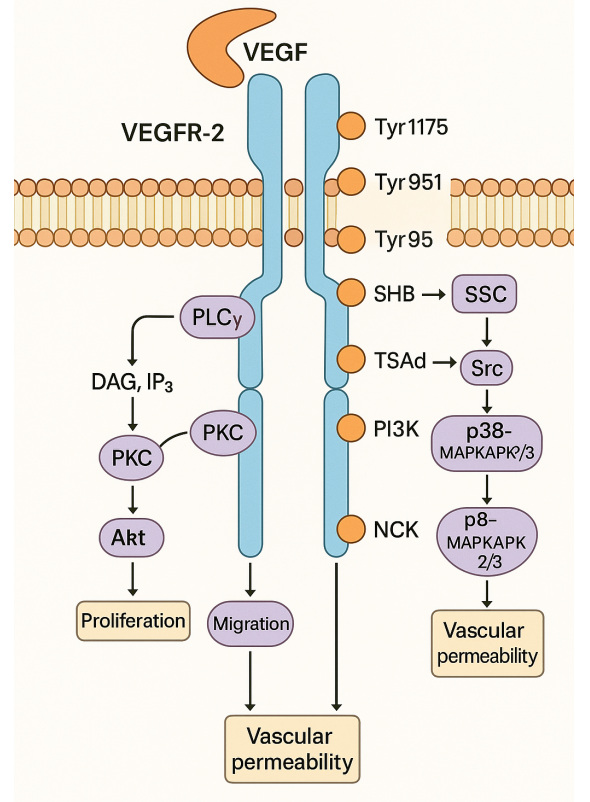
Unlike anti-influenza drugs that target neuraminidase, Baloshavir mapoxilate, as a prodrug, can be rapidly converted into an active form of Baloshavir in vivo. It is by inhibiting influenza virus CAP-dependent endonuclease to inhibit viral replication, in the early stages of influenza virus self-reproduction play drug efficacy, blocking influenza faster than nerve acid inhibitor. Compared with oseltamivir, single administration of Baloshavir is superior to twice daily administration of oseltamivir, and patient compliance will be significantly improved. Therefore, Baloshavir is expected to continue or even surpass the sales of oseltamivir. Baloshavir is also one of the few new drugs in the world that can inhibit the proliferation of influenza viruses.
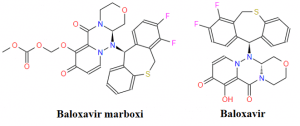
Figure 1. The chemical structure of Baloxavir marboxi and Baloxavir
Action mechanism
Baloxavir marboxi exerts antiviral activity by selectively inhibiting the cap-dependent endonuclease activity of the influenza virus PA protein. PA protein is a subunit of viral RNA polymerase and is necessary for viral RNA transcription. The inhibition of PA protein by Barozavir can block viral replication. The data showed that the median inhibitory concentration (IC50) of Balofloxacin to PA protein of influenza A virus and influenza B virus were 1.4-3.1 nM and 4.5-8.0 nM, respectively.
Pharmacodynamics
Preclinical study
In vitro studies have shown that Baloxavir marboxi has a wide range of pharmacological activities, including against influenza A, B, C, and D viruses. In the viral titer reduction test, the average effective concentration (EC90) of balofloxacin against clinical isolates of influenza A virus (n = 5, H1N1 or H3N2) was 0.63-0.95 nm, and the average effective concentration (EC90) against clinical isolates of influenza B virus (n = 2) was 6.1-6.5 nm. In addition, in the plaque reduction test of 33 clinical isolates, 6 laboratory strains and 12 vaccine strains, the median effective concentration (EC50) of Baloxavir marboxi against influenza A virus (n = 32) was 0.2-1.9 nm, and the median effective concentration (EC50) of influenza B virus (n = 19) was 3.3-13 nm. It is worth noting that the group included influenza A isolates substituted by neuraminidase H274Y, which were resistant to neuraminidase inhibitors oseltamivir and peramivir.
Clinical research
CAPSTONE-1 is a global, multicenter, randomized, double-blind, placebo-controlled phase III study that evaluated the efficacy and safety of Baloshavir. A total of 1436 patients with influenza were included in the trial. Patients were randomly given a single oral dose of 40 mg or 80 mg of Baloshavir (based on body weight), placebo and oseltamivir (75 mg, bid, 5 d). The primary endpoint was the time to symptom relief (OTTAS).
The results showed that compared with the placebo, Baloshavir showed the duration of influenza symptoms was significantly reduced by more than one day (53.7 h vs 80.2 h, P < 0.001); the fever time was significantly shortened (24.5 h vs 42.0 h, P < 0.001). The duration of virus release from the body was reduced (24.0 h vs 96.0 h, P < 0.001). Compared with oseltamivir, Baloshavir showed similar efficacy in reducing the duration of symptoms and fever, but a significant difference was observed in the stopping time of virus excretion (24.0 h vs 72.0 h, P < 0.001).
Pharmacokinetics
Baloxavir marboxil is divided into 20 mg and 40 mg tablets. Once administered, the Cmax usually peaks within 4 hours. When taken with food, the Cmax and AUC decreased by about 48 % and 36 %, respectively. However, Baloshavir can be taken without regard to diet, except for dairy products and products containing positive ions.
After absorption, Baloshavir is almost completely converted to its active metabolite, Baloshavir acid. The protein binding rate of Basalovir was more than 90 %, and the distribution volume was 1180 L. Baloshavir is further metabolized by UGT1A3 to glucuronic acid conjugates, which are then metabolized by CYP3A4 to produce sulfoxide metabolites that are eventually eliminated by bile excretion.
The toleration of Baloxavir marboxil
To date, no specific drug-related adverse events (AEs) have been identified in the study of balofloxacin, despite reports of post-marketing allergies and hypersensitivity reactions, rashes, gastrointestinal disorders, and neuropsychiatric symptoms. In a three-phase randomized controlled trial in other healthy populations, the incidence of AEs (20.7 %) was similar in Baloxavir marboxil recipients to placebo recipients (24.6 %) or oseltamivir recipients (24.8 %), and the incidence of AEs associated with discontinuation of the study drug was 0.3-0.4 % in each group. At least 1 % of AEs reported in adult and adolescent subjects treated with Basalovir included diarrhea (3 %), bronchitis (3 %), nausea (2 %), sinusitis (2 %), and headache (1 %). In children aged 1-11 years, compared with oseltamivir, balofloxacin treatment caused less vomiting (6.1 % vs 15.5 %), but diarrhea was more common (5.2 % vs 1.7 %).
Medication and dosage
In the United States, balofloxacin is used to treat acute, uncomplicated influenza in patients older than 12 years with symptoms less than 48 hours, otherwise healthy, or at high risk of influenza-related complications. Balofloxacin has also been approved in Japan and other countries for the treatment of influenza A or B. The drug is provided in the form of 20 or 40 mg tablets as a single oral dose, and the dose depends on the patient’s body weight: for patients weighing 40-80kg, the recommended dose is 40 mg of balofloxacin (i.e., two tablets 20mg); for patients weighing 80 kg and above, the recommended dose is 80 mg of balofloxacin (i.e., two tablets 40mg). Balosavir should be taken as soon as possible within 48 hours after the onset of influenza symptoms. Should be avoided with dairy products. Calcium-fortified beverages are combined with laxatives, antacids, or oral supplements (such as calcium, iron, magnesium, or zinc supplements) containing multivalent cations.
Drug resistance
In vitro passage and analysis of clinical isolates have confirmed that the substitution of isoleucine at position 38 of the amino-terminal PA domain (PA/I38X) is associated with reduced susceptibility to Baloxavir marboxil. The most common substitution was threonine, but other substitutions were also detected, including methionine, phenylalanine, and leucine. PA/I38X mutations are more common in children than in adults, more common in A/H3N2 influenza than in A/H1N1 influenza, but very rare in influenza B. In a study dominated by A/H1N1, 2.2% of PA/I38X variants were detected in adults treated with balofloxacin. In a study dominated by A/H3N2, 9.7 % of PA/I38X variants were detected in adults and adolescents treated with balofloxacin. In a study dominated by A/H3N2, 23 % of PA/I38X variants were detected in children. The substitution of PA / I38X in clinical A/H3N2 isolates reduced the sensitivity to balofloxacin by 60-155 times in cell culture. The transmissibility of these variants requires close monitoring, particularly in Japan where several cases of primary infection caused by A/H3N2 viruses containing PA/I38T have been documented.
Summary
In summary, the available evidence suggests that taking Baloxavir marboxil within 48 hours of symptom onset is effective in improving influenza symptoms in healthy adolescents and adults, as well as in populations at high risk of influenza complications. Balofloxacin is a type of influenza virus endonuclease inhibitor, which has activity and good tolerance to influenza A and B viruses (including strains resistant to neuraminidase inhibitors). Evidence of the emergence and possible human-to-human transmission of a mutant virus with reduced susceptibility to Baloxavir marboxil highlights the need for continuous monitoring and surveillance. Overall, however, balofloxacin has the advantage of a single-dose oral dosing regimen, providing an effective neuraminidase inhibitor alternative for the treatment of acute non-complicated influenza in adolescents and adults.