Applications of Arylboronic Acids, Not Only in Organic Synthesis
Introduction
Boron (B) occupies the fifth position in the periodic table, making it one of the earliest elements with an atomic number of 5. Despite its small size and only three valence electrons, boron is often perceived as electronically and chemically deficient. This prejudice has obscured the immense potential of boron chemistry, especially in the realm of organoboron compounds.[1] The importance of boron in organic synthesis was recognized through the Nobel Prize awarded to notable chemists, including William N. Lipscomb (1976) for his investigations on the chemical bonding of boranes, Herbert C. Brown (1979) for his work on boron reagents in organic synthesis, and Akira Suzuki (2010) for the development of cross-coupling reactions with organoboron compounds, which greatly impacted medicinal and materials chemistry.[2]
Within the domain of boron organometallics, boronic acids have long been established and well-regarded for their application in organic synthesis, particularly in the Suzuki coupling reaction. The increasing availability of boronic acids on the market has led to their widespread use and popularity in various synthetic endeavors. However, there is a tendency to view boronic acids as important but limited chemicals, primarily serving as intermediates in synthetic chemistry and organometallic catalysis. Nevertheless, the chemistry of boronic acids has extended far beyond the confines of the Suzuki reaction, and its scope is rapidly expanding. Boronic acids have garnered attention in fields such as Medicinal Chemistry, Analytical Chemistry, Materials Science, and Pharmaceutical Chemistry. They find application in diverse areas, including electrochemical sensors, chromatographic columns, electrophoresis gels, polymers, and nanostructures. The versatility of boronic acids is exemplified by numerous examples presented in this review, aiming to draw attention to their multifaceted nature. By doing so, this review hopes to inspire scientists to explore the wide range of new possibilities offered by boronic acids outside the realm of organic synthesis. These compounds are synthetically versatile and possess unique chemical characteristics, such as low toxicity, high stability, tunable Lewis acidity dictated by their substituents, and strong covalent interactions, particularly with biomolecules analogous to diols.
Chemical Properties of Arylboronic Acids
Boronic acids exhibit moderate acidity and can form tetrahedral adducts by coordinating with bases. This is attributed to the electron-deficient nature of the central boron atom, which possesses only six valence electrons due to its sp2 hybridization. Additionally, the boron atom features an empty p orbital that is perpendicular to the molecular plane (Figure 1). Boronic acids act as strong Lewis acids due to the presence of an empty, perpendicular p orbital. As a result, anions readily donate electrons to the B(III) center, leading to the formation of tetrahedral adducts.[3] Boronic acids exhibit strong coordination with water, leading to a reaction that generates a solvated proton and a boronate anion. This process defines a Ka constant. The tetrahedral adducts formed by boronic acids resemble the distinctive configuration of a sp3 carbon atom.
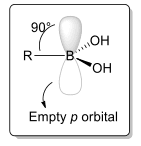
Figure 1 Boronic acid structure
Arylboronic acids possess a wide array of advantageous properties, including high stability, the ability to form oligomeric structures, low toxicity, tunable Lewis acidity, and the presence of an electron-deficient central boron atom. [4] These characteristics have paved the way for a myriad of applications of arylboronic acids across various fields. As a result, there is a growing and significant interest among researchers in exploring and studying these compounds and their systems.
Uses of Arylboronic Acids
In medicinal chemistry
Arylboronic acids have emerged as valuable tools in the field of drug design, with significant implications for therapeutic development. The approval of Velcade (Bortezomib) by the Food and Drug Administration (FDA) in 2003 for treating multiple myeloma, a type of bone marrow cancer, has sparked a surge of interest in synthesizing novel bioactive boronic acids.[5]
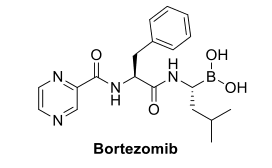
Figure 2 Structure of Bortezomib (Velcade).
According to data from Global Data, Velcade ranked 32nd in terms of pharmaceutical revenue among the top 50 global pharmaceutical products. By 2014, it had achieved a market value exceeding $2 billion. This success has driven the exploration and preparation of various boronic acids with inhibitory activity against enzymes. The B(OH)2 group in these compounds plays a crucial role in their observed activity. This structural requirement arises from the ability of boronic acids to mimic the carboxylic acids found in natural enzyme substrates, effectively acting as transition state analogs during protease reactions (e.g., serine and threonine proteases).
In natural protease reactions, nucleophilic centers (such as the hydroxyl groups of serine and threonine residues) attack the carbonyl of an amide, resulting in the formation of a tetrahedral intermediate. This intermediate subsequently releases the leaving group (R’NH2) and forms an acyl enzyme, which is eventually hydrolyzed. Boronic acids, similar to amides, possess electrophilic centers that can be targeted by nucleophiles within proteases.[6] However, the resulting negatively charged tetrahedral intermediate fails to release the acyl enzyme.
Compared to other electrophiles commonly used in the construction of enzyme inhibitors, such as aldehydes, boronic acids serve as better analogs of the transition state leading to the tetrahedral intermediate. This is due to the negatively charged nature of the boron intermediate, which mirrors the transition state of the natural substrate. Additionally, the release of the boronic inhibitor can be facilitated by pH changes, which is a desirable characteristic.
Furthermore, boronic acids have the ability to establish hydrogen bonds with amino acid side chains present in the active sites of enzymes, facilitating the binding process between the inhibitor and the enzyme. This interaction further enhances the effectiveness of boronic acid-based inhibitors.
In summary, aryl boronic acids have gained prominence in drug design, particularly in the development of enzyme inhibitors. Their ability to mimic transition states, establish hydrogen bonds, and release inhibitors under specific conditions make them versatile and promising compounds in pharmaceutical research.
Supramolecular chemistry
Arylboronic acids offer the advantage of undergoing reversible reactions, making them highly promising for self-assembly processes. Their ability to form compounds with directional characteristics has made them valuable building blocks for the construction of supramolecular assemblies.
The development of nanoporous materials holds significant importance due to their diverse applications, including catalysis, gas storage and separation, energy storage and conversion, optoelectronics, and superhydrophobic interfaces. Reticular chemistry concepts utilize topologically designed building blocks to create networks with discrete pores.[7] Two examples of such nanoporous materials are metal-organic frameworks (MOFs) and covalent organic frameworks (COFs).
In MOFs, an inorganic network is formed by coordinating metals with polytopic linkers. COFs, on the other hand, are crystalline polymers composed of organic building blocks connected through covalent bonds in an ordered manner. These materials have low density due to their composition of lightweight chemical elements (H, B, C, N, O, S), yet exhibit high thermal stability and permanent porosity. The covalent linkages in COFs are synthesized through reversible reactions using dynamic covalent chemistry (DCC), allowing for the correction of misformed bonds and the formation of the most thermodynamically stable structures.
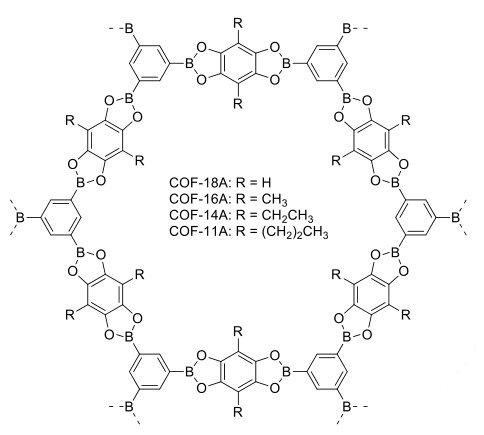
Figure 3 COFs modified with alkyl groups.[8]
The reaction mixtures involved in COF synthesis consist of monomers, oligomers, and polymers that can interconvert with each other under thermodynamic control. Reversible reactions such as the formation of boroxines and boronate esters, as well as imines and hydrazones, are commonly used to construct COFs. It is crucial for the reactions employed in COF construction to be reversible, ensuring the ability to correct and adjust the network’s structure. Furthermore, maintaining the geometry of the building blocks in the final structures is essential.
Arylboronic acids are particularly interesting as building blocks for COFs because they undergo reversible reactions. They can undergo dehydration, resulting in the formation of six-membered anhydrides known as boroxines or condensation with diols to form five-membered boronate esters. The rigidity of the aromatic ring and the directional nature of interactions make arylboronic acids widely used in the construction of COFs.
Nuclear magnetic resonance
Arylboronic acids find significant utility in the field of Nuclear Magnetic Resonance (NMR) spectroscopy. NMR spectroscopy is a powerful analytical technique used to study the structure and dynamics of molecules by measuring the interactions between atomic nuclei and an external magnetic field.
In NMR experiments, arylboronic acids can serve as valuable compounds for several reasons. Firstly, they possess distinctive chemical shifts in proton NMR spectra, typically appearing as broad singlets in the region between 7-10 ppm. This region is often devoid of other proton signals, allowing for easy identification and analysis of arylboronic acids in complex mixtures.
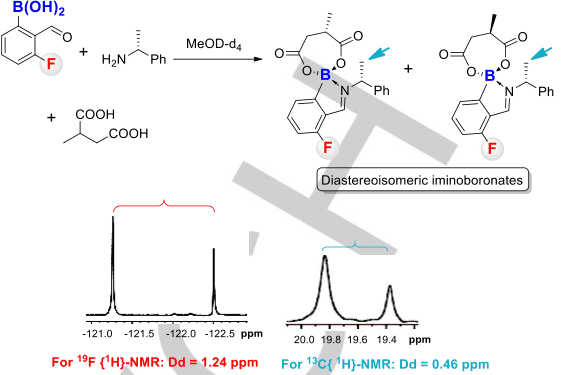
Figure 4 Three-component protocol for discriminating chiral diacids.[8]
Additionally, arylboronic acids exhibit unique characteristics when it comes to coupling with neighboring protons. The coupling patterns observed in NMR spectra provide valuable information about the connectivity and interactions between different atoms in a molecule. In the case of arylboronic acids, the coupling constants between the boron atom and adjacent protons are often small, resulting in weak or no coupling. This phenomenon, known as spin decoupling, simplifies the NMR spectra and facilitates the interpretation of other proton-proton coupling patterns in the molecule.[9]
Arylboronic acids are also known for their ability to form stable complexes with Lewis bases, such as pyridine or DMSO (dimethyl sulfoxide), through coordination to the boron atom. These complexes can exhibit distinctive NMR signals that aid in the characterization and identification of arylboronic acids in solution.
Overall, the unique chemical shifts, spin decoupling properties, and coordination behavior of arylboronic acids make them valuable and versatile compounds in NMR spectroscopy, enabling researchers to study and analyze molecular structures, dynamics, and interactions.
Chemosensors
Arylboronic acids have emerged as important components in the development of chemosensors, which are molecular probes designed to detect and measure the presence of specific analytes in a sample. Chemosensors play a crucial role in various fields, including environmental monitoring, biomedical diagnostics, and pharmaceutical analysis.
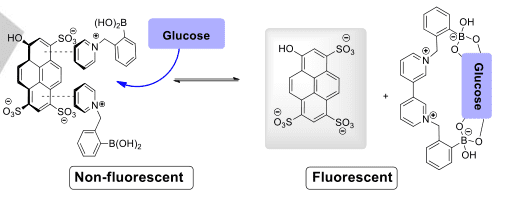
Figure 5 Sensor based on fluorescence restoration [8]
One of the key features of arylboronic acids that makes them attractive for chemosensing applications is their ability to form reversible complexes with diols and polyols through boron-oxygen interactions. This unique property allows arylboronic acids to act as selective receptors for saccharides, including glucose, fructose, and other carbohydrates, which typically contain diol functional groups.
The formation of boronate ester complexes between arylboronic acids and saccharides can lead to noticeable changes in various physicochemical properties, such as absorption or emission of light, fluorescence intensity, or electrochemical behavior. These changes can be easily monitored and quantified, providing a means for the detection and quantification of saccharides in complex biological or environmental samples.[10]
Arylboronic acids have been incorporated into diverse chemosensor platforms, including fluorescent probes, colorimetric sensors, and electrochemical sensors. These sensors can exhibit high selectivity and sensitivity towards specific saccharides, making them valuable tools for glucose monitoring in diabetic patients, for example
Moreover, the versatile nature of arylboronic acids allows for the design and modification of chemosensors to target other analytes beyond saccharides. By modifying the structure of the arylboronic acid moiety, chemosensors can be tailored to recognize and detect various other analytes, such as anions, alcohols, amino acids, and small organic molecules.
In summary, arylboronic acids have proven to be excellent components in the construction of chemosensors due to their reversible complexation with diols and polyols. The incorporation of arylboronic acids in chemosensor platforms enables the selective and sensitive detection of target analytes, opening up avenues for applications in biomedical, environmental, and pharmaceutical fields.
Summary
The review has provided comprehensive evidence of the extensive use and remarkable potential of (aryl)boronic acids. It has effectively demonstrated that the impact of these compounds extends far beyond the realms of organic synthesis and organometallic chemistry. The broad availability of (aryl)boronic acids in the market is expected to facilitate further research and the discovery of numerous additional applications. The chemistry of arylboronic acids has expanded significantly, surpassing initial expectations for such a small and electron-deficient atom like boron (5B). This expansion is rapidly progressing, with notable recognition in various fields, including medicinal chemistry, analytical chemistry, material science, and pharmaceutical chemistry. Furthermore, their unique and versatile chemistry has also made an impact on a diverse range of other disciplines. The presented examples, some of which were discussed in detail throughout the review, serve to emphasize the exceptional versatility of boronic compounds. Despite boron being a small atom with apparent electron deficiency, its compounds exhibit remarkably diverse and significant chemistry. The review highlights that there is still much more to discover and explore in this field, indicating exciting prospects for future research and applications.
Reference
[6] Gluza, K., & Kafarski, P. (2013). Transition state analogues of enzymatic reaction as potential drugs. Drug discovery, 325-372.