What is Ticagrelor? An In-Depth Guide to This Medication
Abstract
Ticagrelor is a reversible P2Y12 receptor antagonist, which means it inhibits the activation of the P2Y12 receptor, a key component in the formation of blood clots. Future research and clinical practice should focus on strategies to improve adherence to Ticagrelor, determine the optimal duration of therapy, investigate the use of Ticagrelor in combination with other agents, and examine the use of Ticagrelor in specific patient populations. Addressing these areas of uncertainty may further optimize the use of Ticagrelor and improve patient outcomes.
What is Ticagrelor?
Ticagrelor is a reversible P2Y12 receptor antagonist, which means it inhibits the activation of the P2Y12 receptor, a key component in the formation of blood clots. (Scheme 1) Ticagrelor is unique among P2Y12 inhibitors in that it binds reversibly to the receptor, allowing for faster recovery of platelet function after discontinuation compared to irreversible inhibitors like clopidogrel. The P2Y12 receptor is primarily found on platelets, small cells in the blood that play a critical role in clotting. Activation of the P2Y12 receptor leads to the aggregation of platelets, which can form a clot and potentially cause a heart attack or stroke. Ticagrelor works by binding to the receptor and blocking its activation, reducing the likelihood of clot formation. Ticagrelor’s reversible binding mechanism confers several advantages, among which its rapid onset of action stands out. Research indicates that Ticagrelor achieves higher levels of platelet inhibition more quickly than other antiplatelet drugs such as clopidogrel. Furthermore, Ticagrelor has demonstrated effectiveness in reducing the risk of cardiovascular events in patients with acute coronary syndrome and, in some instances, has been linked to superior clinical outcomes compared to other antiplatelet agents.
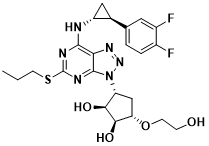
Scheme 1 Structure of Ticagrelor
Pharmacokinetics of Ticagrelor
Understanding the pharmacokinetics of Ticagrelor is important for optimizing its use in clinical practice and minimizing the risk of adverse effects. Ticagrelor has rapid absorption, high plasma protein binding, and is primarily metabolized by CYP3A4.[1] Ticagrelor may interact with other medications that affect platelet function, and caution should be used when co-administering these agents. Ticagrelor’s use in special populations, such as patients with renal or hepatic impairment or in pediatric populations, requires further study.
Absorption and bioavailability:
Ticagrelor is rapidly absorbed after oral administration, with a median time to peak plasma concentration of approximately 1.5 hours. The bioavailability of Ticagrelor is approximately 36%, and its absorption is not affected by food. However, the co-administration of strong CYP3A inhibitors, such as ketoconazole or ritonavir, may increase Ticagrelor exposure and should be avoided.
Distribution and protein binding:
Ticagrelor is extensively distributed throughout the body, with a volume of distribution of approximately 105 L. Ticagrelor has a high plasma protein binding of approximately 99.7%, primarily to albumin. As a result, Ticagrelor may have limited tissue penetration, which could limit its efficacy in certain clinical situations.[2]
Metabolism and elimination:
Ticagrelor is primarily metabolized in the liver by CYP3A4, with minor contributions from CYP3A5 and CYP2C19. The primary metabolite of Ticagrelor is AR-C124910XX[3], which has approximately one-sixth the platelet inhibitory effect of Ticagrelor. Ticagrelor and its metabolites are primarily eliminated through the feces, with approximately 58% of the dose excreted as unchanged Ticagrelor and metabolites.
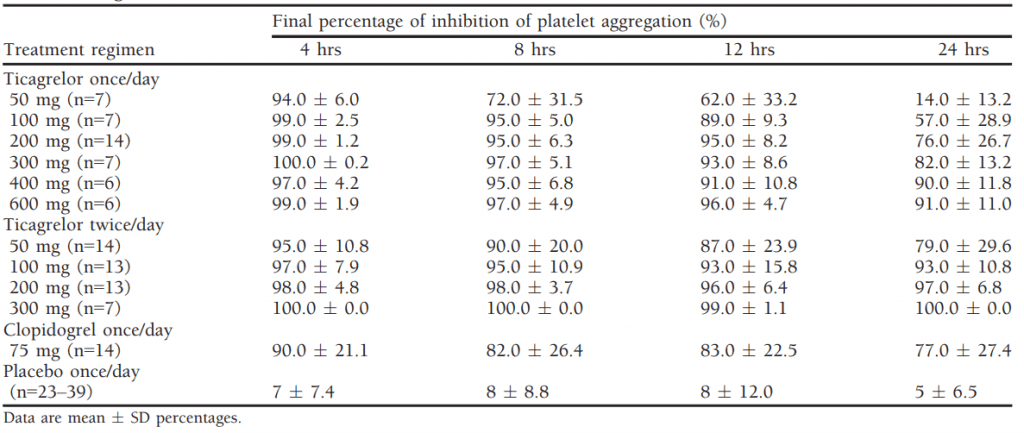
Table 1 Final Percentage of Mean Inhibition of Platelet Aggregation over Time in Healthy Subjects Receiving Multiple Doses of Ticagrelor[1]
Drug-drug interactions:
Ticagrelor is a substrate of CYP3A4 and should be used with caution in patients taking strong CYP3A inhibitors, such as ketoconazole or ritonavir, which may increase Ticagrelor exposure. Conversely, strong CYP3A inducers, such as rifampin or St. John’s wort, may decrease Ticagrelor exposure and should also be used with caution. Ticagrelor may also interact with other medications that affect platelet function, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and anticoagulants, and these combinations should be used with caution due to the increased risk of bleeding.
Special populations:
Extensive research on Ticagrelor in special populations, including elderly patients or those with renal or hepatic impairment, is lacking. Ticagrelor exposure may be elevated in patients with moderate to severe hepatic impairment, and a reduced dosage could be considered. No dose adjustment is necessary for patients with severe renal impairment, despite increased Ticagrelor exposure. However, Ticagrelor’s safety and efficacy in pediatric populations are uncertain since it has not been adequately studied in this group.
Pharmacodynamics of Ticagrelor
Ticagrelor is a potent, reversible, oral P2Y12 receptor antagonist that reduces thrombotic event risk in acute coronary syndrome patients by inhibiting platelet aggregation. Ticagrelor’s quick onset of action comes with an increased risk of bleeding. However, its reversible binding to the P2Y12 receptor allows rapid platelet function recovery post-therapy discontinuation, providing potential benefits in specific clinical scenarios. Ticagrelor’s pharmacodynamic effects may be impacted by genetic variability, but regular genetic testing is not presently advised for Ticagrelor’s use in clinical practice.
Mechanism of action:
Ticagrelor is a reversible, oral P2Y12 receptor antagonist that works by inhibiting platelet aggregation. It exerts its pharmacological effect by binding to the adenosine diphosphate (ADP) binding site on the P2Y12 receptor, which is expressed on the surface of platelets.[4] The P2Y12 receptor is a G protein-coupled receptor that plays a crucial role in platelet activation and aggregation.(Figure 1)
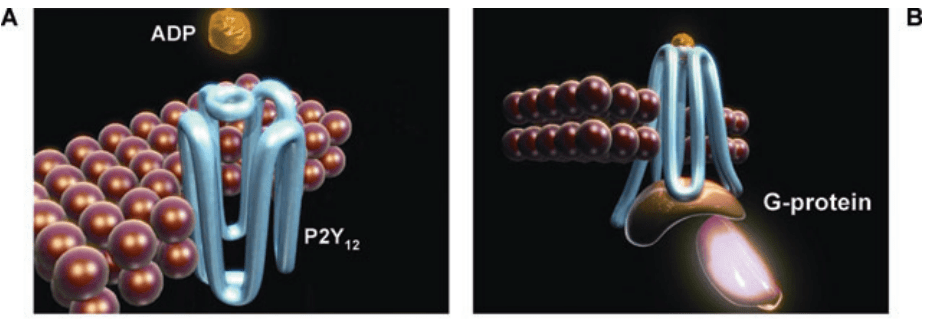
Figure 1 ADP binds to the P2Y12 receptor, resulting in conformational change and G-protein activation[4]
When ADP binds to the P2Y12 receptor, it causes a conformational change in the receptor, leading to the activation of signaling pathways that ultimately result in platelet aggregation. Ticagrelor blocks the binding of ADP to the P2Y12 receptor, thereby inhibiting the activation of the signaling pathways that lead to platelet aggregation. Unlike other P2Y12 receptor antagonists such as clopidogrel, which require metabolic activation to become effective, Ticagrelor is an active drug that binds reversibly to the receptor. (Figure 2) This mechanism of action allows for a rapid onset of action and a predictable pharmacodynamic response.
Additionally, Ticagrelor has been shown to have other mechanisms of action beyond P2Y12 receptor inhibition, such as inhibition of the equilibrative nucleoside transporter 1 (ENT1) and adenosine reuptake, leading to an increase in extracellular adenosine levels. Adenosine is a potent endogenous vasodilator and antiplatelet agent that can help to limit platelet aggregation and thrombus formation.
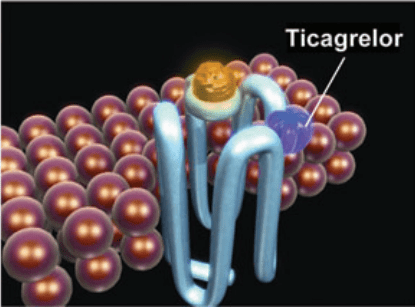
Figure 2 Ticagrelor binds reversibly to P2Y12 at a site distinct from the ADP-binding site and inhibits ADP signaling and receptor conformational change by “locking” the receptor in an inactive state; the receptor is functional after dissociation of the ticagrelor molecule. ADP can still bind at its binding site, and the degree of receptor inhibition (and inhibition of ADP-induced signaling) is dependent on the concentration of ticagrelor. [4]
Platelet inhibition and effect on bleeding time:
Ticagrelor has been shown to be more potent than clopidogrel in inhibiting platelet aggregation, as measured by various platelet function assays. Ticagrelor also has a rapid onset of action, with maximal platelet inhibition achieved within 2 hours of administration. However, the degree of platelet inhibition achieved with Ticagrelor is also associated with an increased risk of bleeding, and this risk should be carefully weighed against the potential benefits of treatment.[5] Ticagrelor has been associated with an increased risk of non-CABG-related major bleeding compared to clopidogrel, and caution should be used in patients at increased risk of bleeding, such as those with a history of bleeding disorders, recent surgery, or concomitant use of anticoagulants.
Platelet function recovery after discontinuation:
Ticagrelor’s reversible binding to the P2Y12 receptor allows for a rapid recovery of platelet function after discontinuation of therapy. Platelet function typically recovers within 24-48 hours after the last dose of Ticagrelor, and this may be an advantage in situations where rapid reversal of the antiplatelet effect is desired, such as in the event of bleeding or the need for emergency surgery.
Genetic variability:
Ticagrelor’s pharmacodynamic effects may be influenced by genetic variability in the P2Y12 receptor and enzymes involved in its metabolism, such as CYP3A4 and CYP2C19. Certain genetic polymorphisms may result in reduced platelet inhibition with Ticagrelor and may be associated with an increased risk of adverse cardiovascular events. However, routine genetic testing is not currently recommended for the use of Ticagrelor in clinical practice.[6]
Clinical efficacy of Ticagrelor
Ticagrelor has demonstrated clinical efficacy in reducing thrombotic events in patients with acute coronary syndrome. Real-world data on its effectiveness and adherence have provided important insights into its clinical use, but caution is advised in patients at increased risk of bleeding. Compared to clopidogrel, Ticagrelor is more effective in reducing cardiovascular events but has a higher risk of bleeding. Prasugrel is also more effective than clopidogrel but has a more limited clinical use. The choice of antiplatelet agent should be individualized based on patient characteristics, clinical context, and the risks and benefits of each agent[7]. Therefore, clinicians should carefully consider each patient’s medical history and current status before prescribing an antiplatelet agent. This personalized approach will help ensure the optimal balance between the prevention of thrombotic events and the risk of bleeding.
Table 2 Efficacy and Safety of Ticagrelor in the Phase III PLATO trial[7]
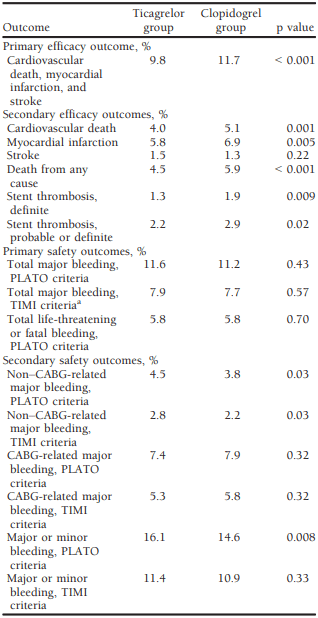
Results of clinical trials in acute coronary syndrome:
Ticagrelor’s efficacy in treating acute coronary syndrome (ACS) has been evaluated through multiple clinical trials. In the PLATO trial, a randomized controlled trial, ticagrelor demonstrated superiority to clopidogrel in reducing the risk of cardiovascular events such as cardiovascular death, myocardial infarction, and stroke. Similarly, the PEGASUS-TIMI 54 trial found that ticagrelor significantly decreased the risk of cardiovascular death, myocardial infarction, and stroke in patients with a history of myocardial infarction, suggesting its effectiveness as an option for ACS treatment. A recent meta-analysis of 17 randomized controlled trials, including over 66,000 patients, confirmed the individual trial results, showing that ticagrelor significantly reduced the risk of cardiovascular events compared to placebo or other antiplatelet agents. The analysis also revealed a favorable safety profile for ticagrelor with a similar rate of bleeding compared to other antiplatelet agents. These trials’ outcomes emphasize ticagrelor’s efficacy and safety in treating ACS, supporting its use as a treatment option for patients with this condition.
Real-world data on effectiveness and adherence:
Real-world studies have provided important insights into the effectiveness and adherence of Ticagrelor, a commonly used antiplatelet agent in patients with cardiovascular disease. According to a meta-analysis of these studies, Ticagrelor demonstrated a lower risk of major adverse cardiovascular events and stent thrombosis compared to clopidogrel. However, the study also found lower adherence to Ticagrelor compared to other antiplatelet agents, which could impact its effectiveness in clinical practice. These findings suggest the need for increased awareness and strategies to improve adherence to Ticagrelor to fully realize its potential benefits in patients with cardiovascular disease.
Comparison with other antiplatelet agents:
In comparison to other antiplatelet agents such as clopidogrel and prasugrel, ticagrelor has shown superior efficacy in reducing the risk of cardiovascular events like myocardial infarction and stroke, but with a higher risk of bleeding[7,8]. Meanwhile, prasugrel has also been found to be more effective than clopidogrel, but with an even greater risk of bleeding and limited clinical use due to its high cost and increased risk of adverse effects in specific patient populations. Choosing an appropriate antiplatelet agent is dependent on individual patient characteristics, including their risk of cardiovascular events and bleeding, as well as their adherence to medication. Despite the greater efficacy of ticagrelor and prasugrel when compared to clopidogrel, these agents may not be suitable for all patients and require careful consideration of potential risks and benefits.
Future directions for research and clinical practice
Despite the proven efficacy and safety of Ticagrelor, there are several areas for future research and clinical practice that could further optimize its use and improve patient outcomes[9].
One area of future research is the development of strategies to improve adherence to Ticagrelor. Low adherence to antiplatelet therapy has been associated with increased risk of thrombotic events, and efforts to improve adherence may help reduce this risk. One potential strategy is the use of novel technologies, such as medication adherence monitoring systems or mobile health applications, to improve patient engagement and adherence to Ticagrelor. Another area of future research is the investigation of the optimal duration of Ticagrelor therapy. Current guidelines recommend at least 12 months of Ticagrelor therapy in patients with acute coronary syndrome, but the optimal duration of therapy beyond this time frame is unclear. Longer-term Ticagrelor therapy may provide continued protection against thrombotic events but may also increase the risk of bleeding. The use of Ticagrelor in combination with other antiplatelet agents or anticoagulants is also an area of future research. The safety and efficacy of combining Ticagrelor with other agents, such as aspirin or direct oral anticoagulants, have not been well studied, and further research is needed to determine the optimal combination and dosing strategies. Finally, the use of Ticagrelor in specific patient populations, such as those with renal impairment or older adults, requires further investigation. The pharmacokinetics and safety of Ticagrelor may differ in these populations, and dosing strategies may need to be adjusted accordingly.