Unraveling Spliceostatin A: How a Natural Compound is Changing RNA and Cancer Research
Abstract
Spliceostatin A is a potent natural compound known for its ability to inhibit pre-mRNA splicing by targeting the SF3b complex within the spliceosome. This blog explores its mechanism of action, therapeutic potential in cancer and neurodegenerative diseases, and its growing role as a molecular tool in RNA biology research. While its clinical application faces challenges due to toxicity and stability issues, ongoing studies on structural analogs like H3B-8800 suggest promising future directions. Spliceostatin A continues to serve as a foundation for precision medicine and innovative therapeutic strategies centered around RNA splicing modulation.
What is Spliceostatin A?
Spliceostatin A is a potent natural product known for its unique ability to inhibit pre-mRNA splicing, a critical step in gene expression. Isolated from Pseudomonas sp. No. 2663, Spliceostatin A belongs to a family of splicing inhibitors that target the spliceosome, the dynamic molecular complex responsible for removing introns from precursor messenger RNA (pre-mRNA) and joining exons to produce mature mRNA. This process is essential for generating functional proteins in eukaryotic organisms.
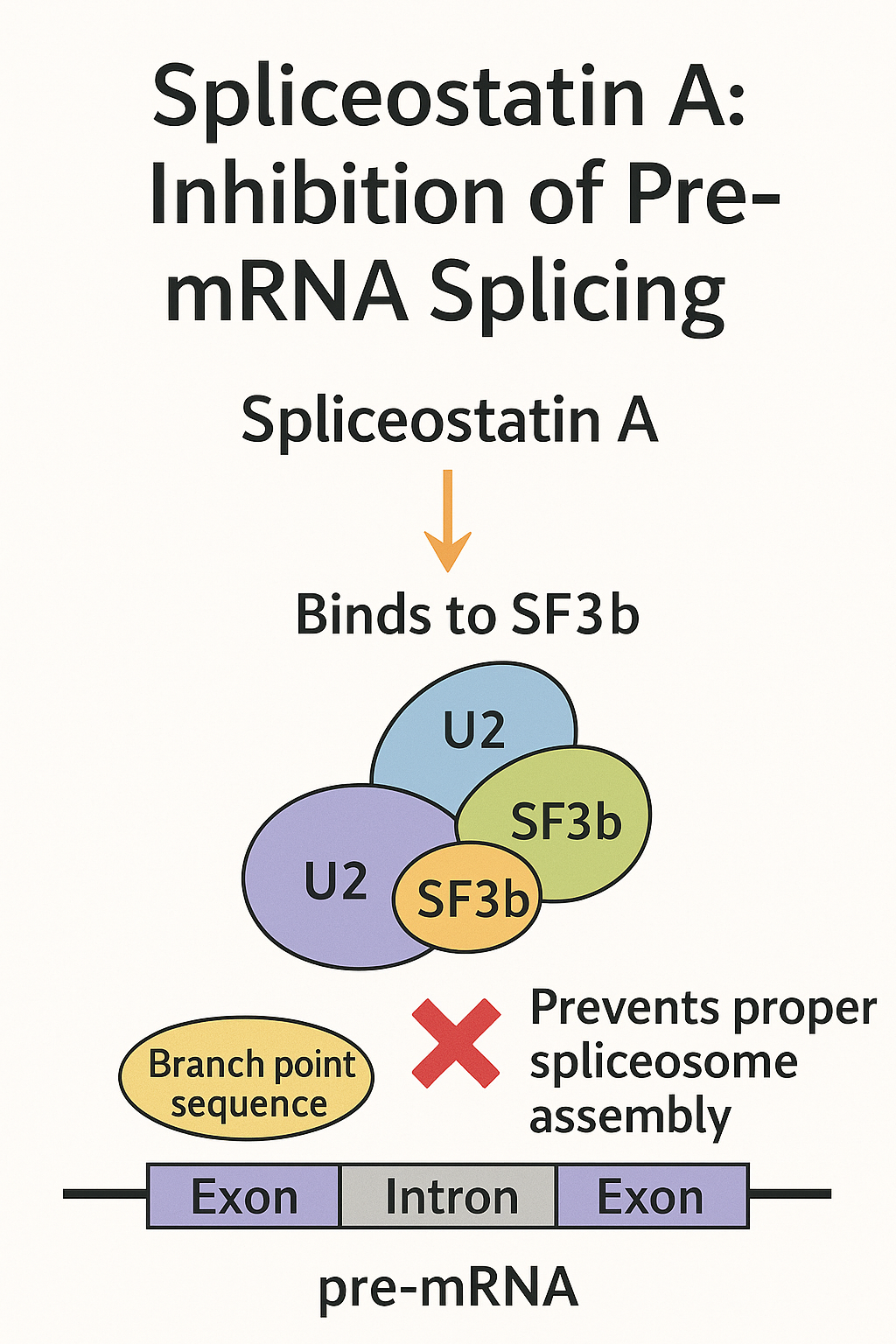
What makes Spliceostatin A particularly interesting to researchers is its mechanism of action. It binds to the SF3b subcomplex of the spliceosome—an essential component of the U2 small nuclear ribonucleoprotein (snRNP). By interfering with the recognition of branch point sequences in introns, Spliceostatin A prevents proper spliceosome assembly, leading to widespread mis-splicing or inhibition of splicing entirely. This disruption can have profound effects on cellular function, particularly in rapidly dividing cells like cancer cells.
Due to its ability to manipulate RNA splicing, Spliceostatin A is widely used as a chemical probe in molecular biology. It helps scientists understand the complexities of spliceosome dynamics and the role of alternative splicing in disease development. Importantly, its application in cancer research is growing, as many tumors exhibit abnormal splicing patterns that could be selectively targeted.
In short, Spliceostatin A is not just a molecular inhibitor—it is a gateway to deeper understanding of gene regulation and a potential tool for novel therapeutic strategies.
The Mechanism of Action of Spliceostatin A
Spliceostatin A exerts its biological effects through a highly specific interaction with the spliceosome, a macromolecular complex responsible for removing non-coding introns from pre-mRNA transcripts. This editing process is essential to produce mature mRNA, which is subsequently translated into functional proteins. The integrity of this splicing process is critical for proper cellular function and gene expression regulation.
The primary target of Spliceostatin A is the SF3b subcomplex within the U2 small nuclear ribonucleoprotein (snRNP). SF3b is essential for recognizing the branch point sequence of introns, a key step in the early stages of spliceosome assembly. By binding to SF3b, Spliceostatin A prevents the proper positioning of U2 snRNP at the branch site, thereby halting the assembly of the functional spliceosome. This blockade causes the splicing reaction to fail, leading to the accumulation of unprocessed or aberrantly spliced pre-mRNA in the nucleus.
Research using in vitro assays and structural biology techniques has confirmed that Spliceostatin A locks the spliceosome in an inactive conformation. This unique mechanism sets it apart from general cytotoxic agents because it selectively targets RNA processing rather than DNA replication or protein synthesis.
The specificity of Spliceostatin A’s action has made it a valuable molecular tool for dissecting splicing mechanisms and identifying vulnerabilities in diseases like cancer, where aberrant splicing plays a pivotal role. Its ability to impair gene expression at the RNA level makes it a promising candidate for precision molecular therapies.
Potential Therapeutic Applications of Spliceostatin A
Spliceostatin A is more than a tool for studying RNA splicing—it holds significant potential as a therapeutic agent, especially in the field of cancer treatment. Because aberrant RNA splicing is a hallmark of many tumors, modulating the splicing machinery has emerged as a novel approach in oncology. Spliceostatin A, through its inhibition of the SF3b complex, disrupts the proper processing of pre-mRNA, leading to selective cytotoxicity in cancer cells.
Studies have demonstrated that Spliceostatin A can inhibit the growth of various cancer cell lines, including leukemia, breast, and colon cancers, by interfering with the production of essential survival and proliferation-related proteins. Cancer cells often rely on specific splicing variants for their growth advantage. By blocking the splicing process, Spliceostatin A effectively undermines this dependency, inducing apoptosis or programmed cell death.
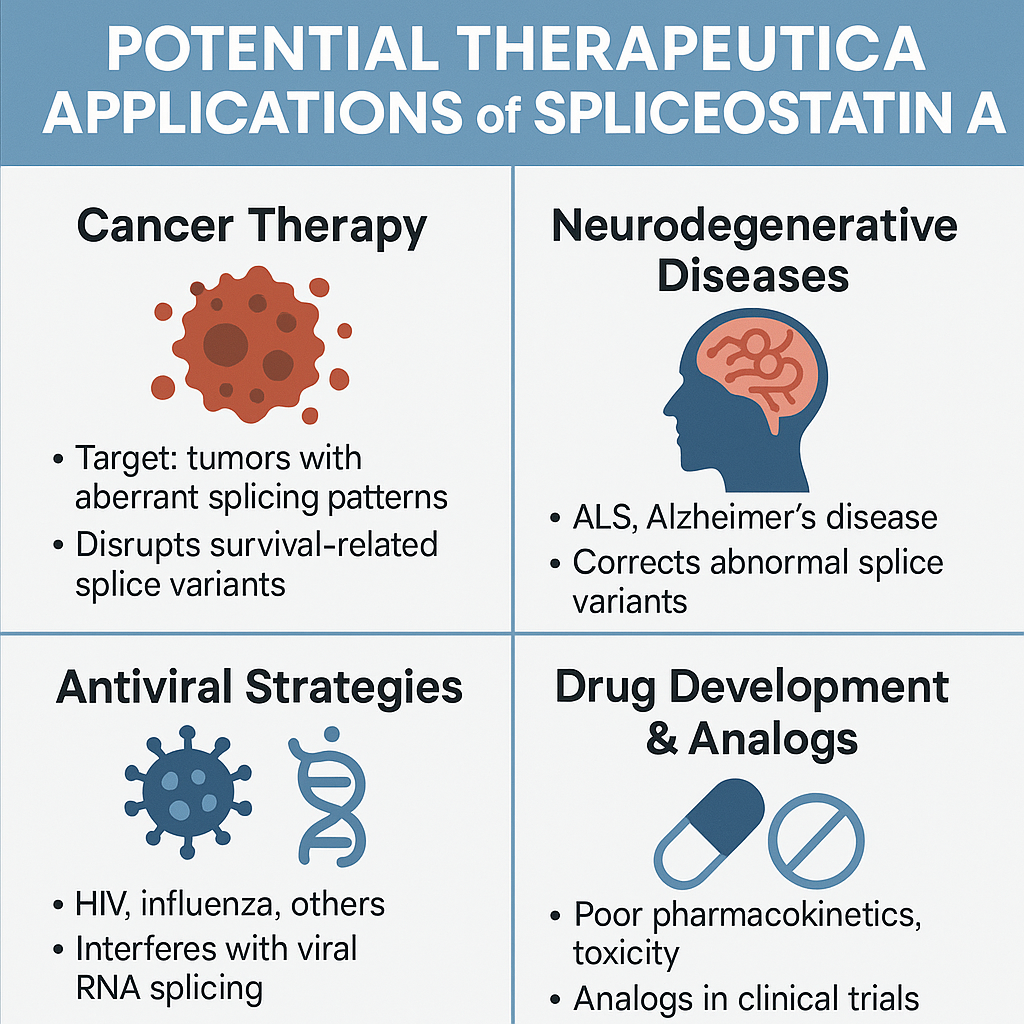
Beyond oncology, researchers are investigating its potential in treating neurodegenerative disorders, such as amyotrophic lateral sclerosis (ALS) and Alzheimer’s disease. Abnormal splicing of genes like TARDBP or MAPT has been implicated in these conditions. Spliceostatin A’s ability to modulate splicing provides a foundation for correcting or suppressing these dysfunctional isoforms.
Additionally, viral infections that exploit the host’s splicing machinery—such as HIV and influenza—could also be susceptible to splicing modulators like Spliceostatin A. By disrupting viral RNA processing, this compound may serve as a broad-spectrum antiviral strategy.
While Spliceostatin A itself has limitations in terms of pharmacokinetics and toxicity, its structural analogs (like pladienolide B and H3B-8800) are already in preclinical and clinical trials, suggesting real promise for splicing-targeted therapeutics in modern medicine.
The Role of Spliceostatin A in Scientific Research
Spliceostatin A has emerged as an indispensable tool in molecular biology research, particularly for studying the mechanisms of RNA splicing and the functional architecture of the spliceosome. Unlike genetic knockdown techniques, Spliceostatin A offers a rapid, reversible, and dose-dependent method to inhibit splicing, making it ideal for dissecting complex, dynamic cellular processes.
One of its most important uses is as a chemical probe to identify how and where splicing regulation fails in diseases like cancer, neurodegeneration, and autoimmune disorders. By blocking the SF3b component of the spliceosome, researchers can observe how cells respond when pre-mRNA processing is abruptly halted. This has led to the discovery of alternative splicing events critical for cell survival, proliferation, and differentiation.
Moreover, Spliceostatin A has helped map the splicing regulatory network, revealing how splicing factors interact and how their malfunction may drive disease. It has also been used in high-throughput screening assays to identify synthetic lethality with other genes—meaning it can help find gene targets that, when inhibited alongside splicing, selectively kill cancer cells.
In virology, Spliceostatin A has been used to explore how viruses hijack the host’s splicing machinery for replication. By disrupting splicing, researchers can impair viral gene expression and identify host–pathogen vulnerabilities.
Additionally, structural biology studies using Spliceostatin A have provided high-resolution snapshots of the spliceosome in inhibited states, helping define the conformational checkpoints essential for its function. These insights are guiding the design of next-generation splicing modulators.
In summary, Spliceostatin A is not just a pharmacological compound—it is a research catalyst driving new discoveries in transcriptomics, therapeutic development, and systems biology.
Challenges and Future Directions for Spliceostatin A
While Spliceostatin A offers valuable insights into RNA splicing and holds promise for therapeutic development, its path toward clinical application faces several critical challenges. Chief among these are toxicity, stability, and delivery issues. As a natural product with a complex macrocyclic structure, Spliceostatin A is chemically unstable and prone to degradation under physiological conditions. This limits its usefulness in in vivo settings and hinders formulation for clinical trials.
Another major limitation is its lack of specificity for diseased versus healthy cells. Because RNA splicing is a ubiquitous and essential cellular process, systemic inhibition can lead to undesirable effects in non-target tissues. This presents a narrow therapeutic window, necessitating either improved targeting methods or the development of analogs with higher specificity.
To address these issues, researchers have turned to structurally related derivatives like pladienolide B and H3B-8800, which show better pharmacokinetic profiles and are already progressing through preclinical and clinical pipelines. These compounds aim to exploit splicing vulnerabilities in cancer cells—such as mutations in splicing factors like SF3B1 or SRSF2—that make them more sensitive to splicing inhibitors.
Looking ahead, the future of Spliceostatin A and its analogs lies in precision medicine, where personalized splicing profiles could help identify patients most likely to benefit from such therapies. Additionally, combining splicing modulators with other treatments—like immunotherapy or kinase inhibitors—may enhance effectiveness while reducing systemic toxicity.
In parallel, ongoing structural studies and high-throughput screenings are expected to yield next-generation splicing inhibitors that are more selective, potent, and safer. Spliceostatin A has laid the groundwork; now, innovation must carry it forward into real-world therapeutics.
References
Kaida, D., Motoyoshi, H., Tashiro, E., Nojima, T., Hagiwara, M., & Yoshida, M. (2007). Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre-mRNA. Nature Chemical Biology, 3(9), 576–583.
https://www.nature.com/articles/nchembio.2007.18
Kotake, Y., Sagane, K., Owa, T., Mimori-Kiyosue, Y., Shimizu, H., Uesugi, M., & Ishihama, Y. (2007). Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nature Chemical Biology, 3(9), 570–575.
https://www.nature.com/articles/nchembio.2007.16
Corrionero, A., Minana, B., & Valcarcel, J. (2011). Reduced fidelity of branch point recognition and alternative splicing induced by the anti-tumor drug Spliceostatin A. Genes & Development, 25(5), 445–459.
https://genesdev.cshlp.org/content/25/5/445.short
Effenberger, K. A., Urabe, V. K., & Jurica, M. S. (2017). Modulating splicing with small molecular inhibitors of the spliceosome. Wiley Interdisciplinary Reviews: RNA, 8(2), e1381.
https://wires.onlinelibrary.wiley.com/doi/abs/10.1002/wrna.1381
Seiler, M., Yoshimi, A., Darman, R., Chan, B., Keaney, G., Thomas, M., … & Abdel-Wahab, O. (2018). H3B-8800, an orally available small-molecule splicing modulator, induces lethality in spliceosome-mutant cancers. Nature Medicine, 24(4), 497–504.
https://www.nature.com/articles/nm.4493
Bonnal, S. C., López-Oreja, I., & Valcárcel, J. (2020). Roles and mechanisms of alternative splicing in cancer—implications for care. Nature Reviews Clinical Oncology, 17(8), 457–474.