Tirzepatide and the Brain: A Breakthrough in Combating Diabetes-Linked Neurodegeneration
Abstract
Tirzepatide, a novel dual agonist of the GIP and GLP-1 receptors, has gained attention for its superior efficacy in managing type 2 diabetes mellitus (T2DM). Recent research now highlights its potential to counteract neurodegenerative processes associated with chronic hyperglycemia and insulin resistance—factors closely linked to cognitive decline and Alzheimer’s disease. This blog explores findings from a 2024 study demonstrating Tirzepatide’s ability to activate the pAkt/CREB/BDNF signaling pathway, reduce neuronal apoptosis, promote differentiation, and restore glucose transporter function in high-glucose-exposed neuronal cells. Importantly, Tirzepatide also reverses harmful epigenetic changes, including DNA methylation and microRNA dysregulation, suggesting long-term neuroprotective potential. These multifaceted actions place Tirzepatide at the intersection of metabolic and neurological therapy, offering a promising approach to preventing diabetes-associated cognitive impairment and potentially delaying the onset of neurodegenerative diseases.
Introduction: A New Frontier in Diabetes and Brain Health
For decades, managing type 2 diabetes mellitus (T2DM) has primarily focused on blood sugar control. However, an emerging body of research reveals a deeper and more complex link between diabetes and cognitive decline. Individuals with T2DM face a significantly higher risk of developing neurodegenerative disorders such as Alzheimer’s disease (AD), prompting researchers to explore therapies that offer both metabolic and neurological protection.
Tirzepatide, a recently developed dual agonist of the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors, is at the forefront of this paradigm shift. Originally designed as a next-generation antidiabetic drug, Tirzepatide has demonstrated superior glycemic control compared to existing GLP-1 receptor agonists like semaglutide. Yet its potential extends well beyond blood sugar management.
A 2024 study published in the Journal of Translational Medicine has uncovered Tirzepatide’s ability to counteract neuronal damage caused by high glucose levels. This research reveals that Tirzepatide not only enhances insulin sensitivity but also activates crucial molecular pathways in brain cells that are involved in growth, survival, and repair. These findings open up new possibilities for using metabolic drugs to prevent or even reverse the neurological damage associated with diabetes.
The brain, once thought to be insulin-independent, is now recognized as a highly insulin-sensitive organ, especially vulnerable in the context of chronic hyperglycemia. When insulin signaling in the brain falters—as it often does in T2DM—neurons suffer from impaired glucose uptake, increased oxidative stress, and reduced synaptic plasticity. These disruptions contribute to the hallmark features of dementia, including memory loss and cognitive decline.
Tirzepatide’s emerging neuroprotective profile invites us to rethink traditional diabetes therapies. Could this dual receptor agonist represent a bridge between metabolic and cognitive health? As research progresses, this question becomes increasingly relevant not only for diabetics but for the broader fight against neurodegenerative diseases.
The Science Behind Cognitive Decline in Diabetes
The relationship between type 2 diabetes mellitus (T2DM) and cognitive impairment is no longer viewed as coincidental. Extensive research now confirms that chronic hyperglycemia and insulin resistance play a central role in accelerating neurodegenerative processes, particularly those seen in Alzheimer’s disease (AD). This has led to the hypothesis of “type 3 diabetes” — a term used to describe AD as a brain-specific manifestation of insulin resistance.
In the healthy brain, insulin facilitates glucose uptake, supports neuronal survival, and promotes synaptic plasticity—essential for memory formation and learning. However, in T2DM, impaired insulin signaling disrupts these processes. Neurons become less responsive to insulin, glucose transport is reduced, and energy deficits ensue. Over time, this contributes to synaptic failure, oxidative stress, and even tau protein hyperphosphorylation—key pathological features of AD.
One of the most critical signaling pathways affected by insulin resistance in the brain is the PI3K/Akt/CREB/BDNF cascade. CREB (cAMP response element-binding protein) is a transcription factor that governs the expression of BDNF (brain-derived neurotrophic factor), a neurotrophin vital for neuronal growth, differentiation, and synaptic maintenance. When glucose metabolism is disrupted, this pathway is suppressed, leading to neuronal atrophy and cognitive decline.
Furthermore, glucose transporter proteins (GLUTs) are essential for maintaining neuronal glucose homeostasis. GLUT1 and GLUT3 are the primary glucose transporters in the brain, while GLUT4, though more prominent in peripheral tissues, also plays a role in the hippocampus. In the context of diabetes and AD, the downregulation of these transporters has been consistently observed, resulting in reduced glucose availability to neurons and subsequent degeneration.
These mechanistic overlaps between diabetes and Alzheimer’s have sparked interest in repurposing antidiabetic therapies for neurodegenerative conditions. Agents that improve insulin sensitivity, enhance glucose transport, and stimulate CREB-BDNF signaling—such as Tirzepatide—are now being explored for their dual benefits in both metabolic and cognitive health.
Tirzepatide’s Protective Role: Key Molecular Pathways
Recent findings reveal that Tirzepatide, a dual GIP and GLP-1 receptor agonist, may offer neuroprotective benefits far beyond its original indication for blood glucose control in type 2 diabetes mellitus (T2DM). In a 2024 study using human neuroblastoma SHSY5Y cells, researchers explored how Tirzepatide influences molecular pathways that govern neuronal growth, survival, and function—especially under high glucose (HG) stress that mimics diabetic conditions.
One of the most significant findings is Tirzepatide’s activation of the pAkt/CREB/BDNF signaling cascade. This pathway is essential for neuronal plasticity, memory formation, and resistance to neurodegenerative insults. High glucose levels were shown to suppress this cascade, leading to reduced expression of CREB (a key transcription factor) and BDNF (a critical neurotrophin). Tirzepatide reversed these effects, restoring both mRNA and protein levels of CREB and BDNF, even in the presence of glucose-induced stress. This suggests that Tirzepatide promotes neuronal resilience and may help preserve cognitive function in diabetics at risk for dementia.
Beyond neurotrophic support, Tirzepatide also exerts anti-apoptotic effects. It reduced the expression of BAX (a pro-apoptotic protein) and increased Bcl-2 (an anti-apoptotic counterpart), lowering the BAX/Bcl-2 ratio—an established marker of cell death susceptibility. These effects indicate that Tirzepatide may protect neurons from glucose-induced apoptosis, a hallmark of neurodegenerative processes.
Tirzepatide further promoted neuronal differentiation and regeneration. It significantly upregulated markers such as MAP2, GAP43, and AGBL4, all of which are associated with neuronal development, axonal growth, and synaptic remodeling. These proteins are particularly important in maintaining brain structure and function in aging or diseased states.
Lastly, Tirzepatide enhanced the expression of glucose transporters (GLUT1, GLUT3, and GLUT4) and SORBS1, a protein involved in insulin-stimulated glucose uptake. This effect is vital because impaired glucose transport is a major contributor to neuronal dysfunction in both diabetes and Alzheimer’s disease.
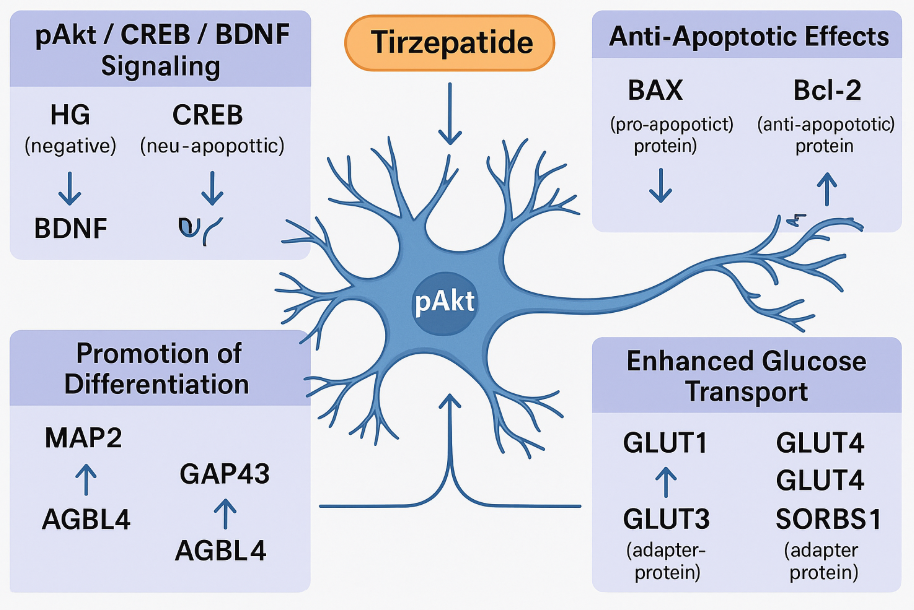
Fig.1 Tirzepatide Protects Neurons: A Molecular Map of Its Neuroprotective Actions
In sum, Tirzepatide simultaneously supports neuronal growth, prevents cell death, and improves energy metabolism—a rare combination that positions it as a promising therapeutic candidate for metabolic neurodegeneration.
Epigenetic Insights: Tirzepatide Rewrites the Brain’s Response
One of the most compelling aspects of Tirzepatide’s neuroprotective effect lies in its ability to influence epigenetic mechanisms—molecular changes that regulate gene expression without altering the DNA sequence. These mechanisms are increasingly recognized as key players in both the development and progression of neurodegenerative diseases, particularly those linked to metabolic disorders like type 2 diabetes mellitus (T2DM).
In conditions of chronic hyperglycemia, such as those modeled in this study using high glucose-exposed SHSY5Y neuronal cells, epigenetic changes are prominent. The 2024 study by Fontanella et al. revealed that high glucose increased DNA methylation in the promoter regions of CREB and BDNF, two genes critical for neuronal growth and synaptic plasticity. Methylation in these regions typically reduces gene expression, contributing to impaired learning, memory loss, and neuronal atrophy.
Importantly, treatment with Tirzepatide reversed these methylation changes, restoring the transcriptional activity of CREB and BDNF. This epigenetic reprogramming allows the reactivation of neuroprotective pathways, even under persistent metabolic stress, suggesting that Tirzepatide can reshape the neuronal epigenetic landscape in favor of survival and regeneration.
In addition to DNA methylation, the study highlighted Tirzepatide’s impact on microRNA (miRNA) regulation. miRNAs are short, non-coding RNAs that fine-tune gene expression post-transcriptionally. Under high glucose conditions, miR-34a, known for its association with aging and neurodegeneration, was significantly upregulated. Tirzepatide treatment suppressed miR-34a, while upregulating miR-212 and miR-29c, both of which support anti-apoptotic and pro-differentiation signaling pathways. These miRNAs also modulate the PI3K/Akt/CREB axis, reinforcing the molecular benefits observed in earlier parts of the study.
Together, these findings suggest that Tirzepatide does more than temporarily modify protein levels—it resets the epigenetic switches that control long-term neuronal fate. This positions the drug as a promising candidate for disease-modifying strategies in neurodegeneration linked to diabetes.
Future Outlook: Could Tirzepatide Help Prevent Alzheimer’s?
With growing global concern about the rise in both type 2 diabetes mellitus (T2DM) and neurodegenerative diseases such as Alzheimer’s disease (AD), there is an urgent need for therapies that address the metabolic-cognitive connection. Tirzepatide, a dual GIP/GLP-1 receptor agonist, may represent a new generation of drugs that can do just that—treat metabolic dysfunction while preventing or slowing neurodegeneration.
The compelling in vitro findings presented by Fontanella et al. (2024) position Tirzepatide as more than a blood sugar regulator. By restoring glucose transporter expression, reducing apoptosis, promoting neuronal growth, and reversing harmful epigenetic changes, Tirzepatide targets the underlying mechanisms that link diabetes to cognitive decline. These multifaceted effects suggest that Tirzepatide could be repurposed as a disease-modifying treatment for early-stage neurodegeneration, especially in diabetic or prediabetic patients.
Though promising, these conclusions are still limited to cellular models. To determine Tirzepatide’s true therapeutic potential in the human brain, robust clinical studies in cognitively impaired diabetic populations are needed. Previous studies with GLP-1 receptor agonists such as liraglutide have shown cognitive improvements in both animals and humans, and now Tirzepatide may go a step further, offering dual incretin signaling that more comprehensively regulates neuronal metabolism and survival.
There is also growing interest in the preventive use of such agents. If started early—potentially even in midlife—Tirzepatide could delay or reduce the onset of cognitive symptoms in at-risk individuals, especially those with metabolic syndrome, insulin resistance, or early T2DM. This approach could shift the paradigm from symptom management to proactive neuroprotection, aligning with current goals in both neurology and endocrinology.
In conclusion, Tirzepatide exemplifies the emerging class of therapeutics that blur the line between metabolic and neurological medicine. As our understanding of the brain–body axis deepens, drugs like Tirzepatide could become key tools not just for managing diabetes, but for rewriting the course of neurodegenerative disease.
References
Fontanella, R. A., Ghosh, P., Pesapane, A., Taktaz, F., Puocci, A., Franzese, M., … & Barbieri, M. (2024). Tirzepatide prevents neurodegeneration through multiple molecular pathways. Journal of Translational Medicine, 22(114).
https://doi.org/10.1186/s12967-024-04927-z
Butterfield, D. A., Di Domenico, F., & Barone, E. (2014). Elevated risk of type 2 diabetes for development of Alzheimer disease: A key role for oxidative stress in brain. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease, 1842(10), 1693–1706.
https://doi.org/10.1016/j.bbadis.2014.06.010
Ghosh, P., Fontanella, R. A., Scisciola, L., Pesapane, A., Taktaz, F., Franzese, M., … & Barbieri, M. (2023). Targeting redox imbalance in neurodegeneration: Characterizing the role of GLP-1 receptor agonists. Theranostics, 13(10), 4872–4884.
https://doi.org/10.7150/thno.86831
Norgaard, C. H., Friedrich, S., Hansen, C. T., Gerds, T. A., Ballard, C., Moller, D. V., … & Holm, E. (2022). Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: Data from pooled double-blind randomized controlled trials. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 8, e12268.
https://doi.org/10.1002/trc2.12268
Lesort, M., Jope, R. S., & Johnson, G. V. (1999). Insulin transiently increases tau phosphorylation: Involvement of glycogen synthase kinase-3β and Fyn tyrosine kinase. Journal of Neurochemistry, 72(2), 576–584.
https://doi.org/10.1046/j.1471-4159.1999.0720576.x
Scisciola, L., Chianese, U., Caponigro, V., Basilicata, M. G., Salviati, E., Altucci, L., … & Sommella, E. (2023). Multi-omics analysis reveals attenuation of cellular stress by empagliflozin in high glucose-treated human cardiomyocytes. Journal of Translational Medicine, 21, 662.
https://doi.org/10.1186/s12967-023-04537-1
Yonamine, C. Y., Passarelli, M., Suemoto, C. K., Pasqualucci, C. A., Jacob-Filho, W., Alves, V. A. F., … & Machado, U. F. (2023). Post-mortem brains from subjects with diabetes mellitus display reduced GLUT4 expression and soma area in hippocampal neurons: Potential involvement of inflammation. Cells, 12(9), 1250.
https://doi.org/10.3390/cells12091250
Franzese, M., … & Barbieri, M. (2024). Tirzepatide prevents neurodegeneration through multiple molecular pathways. Journal of Translational Medicine, 22(114).
https://doi.org/10.1186/s12967-024-04927-z
Li, C., Sui, C., Wang, W., Yan, J., Deng, N., Du, X., … & Wang, Q. (2021). Baicalin attenuates oxygen-glucose deprivation/reoxygenation-induced injury by modulating the BDNF-TrkB/PI3K/Akt and MAPK/Erk1/2 signaling axes in neuron-astrocyte cocultures. Frontiers in Pharmacology, 12, 599543.
https://doi.org/10.3389/fphar.2021.599543
Riccio, A., Ahn, S., Davenport, C. M., Blendy, J. A., & Ginty, D. D. (1999). Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science, 286(5448), 2358–2361.
https://doi.org/10.1126/science.286.5448.2358
Sarkar, S., Jun, S., Rellick, S., Quintana, D. D., Cavendish, J. Z., & Simpkins, J. W. (2016). Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Research, 1646, 139–151.
https://doi.org/10.1016/j.brainres.2016.05.026
Remenyi, J., Hunter, C. J., Cole, C., Ando, H., Impey, S., Monk, C. E., … & Arthur, J. S. (2010). Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochemical Journal, 428(2), 281–291.
https://doi.org/10.1042/BJ20100024
Guo, X., Lei, M., Zhao, J., Wu, M., Ren, Z., Yang, X., … & Chen, Q. (2023). Tirzepatide ameliorates spatial learning and memory impairment through modulation of aberrant insulin resistance and inflammation in diabetic rats. Frontiers in Pharmacology, 14, 1146960.