The Role of TIM3 Immune Checkpoint in Cancer Immunotherapy
Abstract
TIM3 is a protein receptor expressed on immune cells like T cells and NK cells. It interacts with its ligand, galectin-9, to regulate immune responses. In normal conditions, TIM3 helps maintain immune homeostasis by dampening T cell and NK cell activity. However, TIM3 dysregulation is implicated in diseases like cancer and autoimmune disorders. Blocking TIM3 signaling is a promising approach in cancer immunotherapy, as it restores exhausted T cell function and enhances anti-tumor immune responses. Clinical studies are underway to evaluate TIM3-targeted therapies, showing encouraging results, though further research is needed to optimize treatments and understand the interplay with other immune checkpoints.
Tumor immunotherapy has become very popular in recent years. TIM-3 is another emerging immune checkpoint molecule after PD-1/PD-L1 and CTLA-4.
As reflected in the name of TIM-3, the current research on it mainly focuses on T cells, and TIM-3 is regarded as a marker molecule of T cell exhaustion in chronic viral infection and cancer models.
Although several drugs targeting TIM-3 have entered clinical trials, the mechanism of blocking TIM-3’s anti-tumor activity is still somewhat unclear, because TIM-3 is also expressed on many other immune cells, and immune cells other than T cells Are cells involved in its anti-cancer effects, or even more important?
Introduction to TIM-3
TIM3 (T cell immunoglobulin and mucin domain molecule 3), also known as HAVCR2, is a member of the TIM family of immunomodulatory proteins. In humans, the TIM family includes TIM1, TIM3 and TIM4, and is located on chromosome 5q33.2. In mice, the TIM family includes TIM1 to TIM8, located on chromosome 11B1.1. A large body of evidence shows that the TIM family plays different roles in the regulation of immune responses such as autoimmune diseases, infectious diseases, tumor immune surveillance and immune escape. TIM3 is a class of T cell surface inhibitory molecules that can cause T cell exhaustion during cancer and chronic viral infection. Similar to CTLA4 and PD1, it is also one of the most studied immunotherapy targets.
As a negative regulatory immune checkpoint, TIM3 was first discovered in 2002, consists of 281 amino acids, and consists of an extracellular domain, a single transmembrane domain and a C-terminal cytoplasmic tail. TIM3 is selectively expressed in IFN-γ-secreting helper T cells (Th1 and Th17), regulatory T cells (Treg), dendritic cells (DCs), monocytes, mast cells, NK cells, and tumor infiltrating lymphocytes. Cells (TILs) are also expressed on tumor cells, such as melanoma, gastric cancer, and B-cell lymphoma. The mechanism by which TIM3 acts as an important immune checkpoint lies in the fact that TIM3 marks the most dysfunctional subset of tumor-infiltrating CD8+PD1+ T cells. Antibodies that simultaneously block TIM3 and PD1 pathways have synergistic effects on inhibiting tumor growth and improving tumor antigen-specific CD8+ T cell responses.
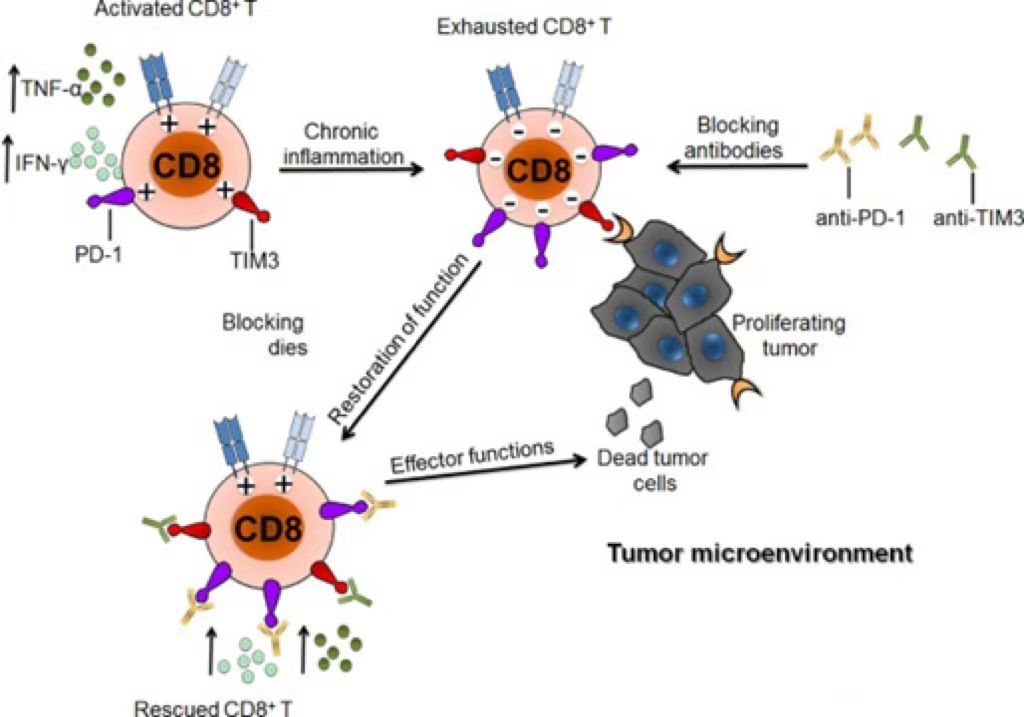
Figure 1: TIM3 can induce T cell exhaustion
TIM-3 is known to have a variety of ligands, such as phosphatidylserine (PtdSer), S-type clusterin galectin-9 (Galectin-9), high mobility group box B-1 (HMGB1) and carcinoembryonic antigen Related cell adhesion molecule 1 (Ceacam-1). Among them, the interaction between TIM-3 and PtdSer can promote the clearance of apoptotic bodies in the TME; the interaction between TIM-3 and Gal-9 will inhibit the activity of T cells and make T cells appear “exhausted”, thereby regulating T cells Apoptosis and immune tolerance; HMGB1 binds DNA released from dying cells and promotes delivery of innate immune cells by binding to advanced glycation end products (RAGE) and Toll-like receptors (TLR), thereby triggering innate immune cells Activation and production of pro-inflammatory cytokines. Binding of Tim-3 to HMGB1 interferes with this process, thereby inhibiting the activation of the innate immune response.
TIM-3 structure and function
TIM-3 consists of three parts: extracellular region, transmembrane region and intracellular region. where the extracellular domain consists of an N-terminal extracellular immunoglobulin variable region (IgV domain) with an FG-CC’ loop and N-linked glycans, a mucin domain containing O-linked glycosylation sites, and It consists of a stalk domain containing N-linked glycans, the transmembrane region consists of a transmembrane region, and the intracellular region consists of a cytoplasmic tail with five tyrosine residues. The IgV domain contains the binding site for its ligand. Phosphatidylserine (Ptdser), carcinoembryonic antigen-associated cell adhesion molecule (CEACAM1), and high mobility group box 1 (HMGB1) bind to the FG-CC’ loop, while Gal9 binds to N-linked glycans.
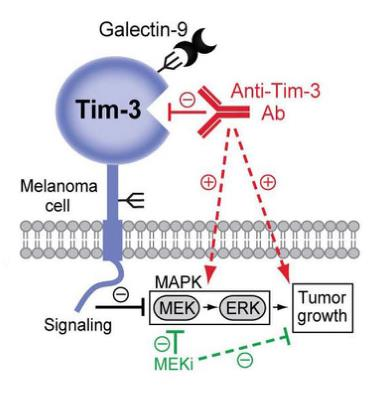
Figure 2: Structure of TIM3
TIM-3 target mechanism of action
TIM-3, as an immune checkpoint protein, regulates the exhaustion of CD8+ T cells together with other immune checkpoints such as PD-1 and LAG3. TIM3, also a CD4+Th1-specific cell surface protein, can regulate macrophage activation and enhance the severity of experimental autoimmune encephalomyelitis in mice.
TIM3 has four ligands, including galectin 9 (Gal-9), carcinoembryonic antigen cell adhesion molecule 1 (CEACAM-1), high mobility group box B1 (HMGB1) and phosphatidylserine (PtdSer). Gal-9 is the main ligand, and all four ligands interact with the IgV domain of TIM-3:
The binding of TIM3 to its main ligand Gal9 leads to the death of helper T cells (Th1/Th17) by apoptosis.
In the tumor microenvironment (TME), TIM3 expressed by tumor-associated dendritic cells binds to high-mobility group box 1 (HMGB1) molecules released by apoptotic tumor cells. This interaction blocks the transport of nucleic acids to dendritic cell endosomes, thereby suppressing the innate immune response to tumor-derived nucleic acids normally mediated by dendritic cells.
Upregulation of TIM3 in mature natural killer cells often induces the release of cytokines IL-12, IL-15 and/or IL-18 and increases tumor cell cytotoxicity.
In CD8+ T cells, the expression of TIM3 and other inhibitory immune checkpoint molecules such as PD-1, CD160; 2B4, LAG3, etc. are considered to be related to T cell differentiation and activation, with increased levels of IFN-γ and TNF-α related. And blocking the TIM3 pathway may inhibit Treg activation.
The affinity and functions of TIM3 to all its ligands have not been fully studied.
TIM-3 signaling pathways
The TIM-3 signaling pathway comprises a complex network of molecular interactions involving multiple proteins and signaling molecules. TIM-3 is a protein expressed on the surface of T cells, and it can interact with its ligand, Galectin-9, resulting in various downstream effects.
One of the critical roles of TIM-3 is to regulate T cell activation and function. Upon binding to Galectin-9, TIM-3 can hinder T-cell proliferation, cytokine production, and cytotoxic activity. This can result in the attenuation of immune responses, which can be advantageous in some contexts such as curbing autoimmunity or reducing inflammation.
However, TIM-3 can also play a role in promoting immune exhaustion, particularly in chronic infections or cancer. In these settings, sustained expression of TIM-3 can lead to T cell dysfunction and exhaustion, which can impair the ability of the immune system to clear the infection or fight cancer.
The downstream signaling pathways activated by TIM-3 are complex and can involve a range of intracellular signaling molecules, including Src family kinases, phosphatases, and adaptor proteins. The precise mechanisms by which TIM-3 regulates T cell function and exhaustion are still being elucidated, and ongoing research is aimed at developing new therapies that target this pathway to enhance immune responses and improve outcomes in a range of diseases.
The role of TIM-3 in tumor targeting therapy
TIM-3 is expressed in a variety of T cells and is expected to become a target for tumor therapy. TIM-3 is also expressed on myeloid cells such as dendritic cells, macrophages and monocytes. TIM-3 plays an important role in the anti-tumor immune response mediated by innate immune cells.
An increasing number of preclinical studies have reported that TIM-3 can enhance the efficacy of cancer immunotherapy.
In preclinical studies, TIM-3 inhibitors have shown similar effects to PD-1 inhibitors. It has been reported that PD-1 antibody can lead to increased expression of TIM-3 in an in vivo model of lung cancer, suggesting that TIM-3 may be one of the hallmarks of PD-1 blocking antibody resistance. In HCC tissues, the expressions of PD-1, TIM-3, and LAG-3 were upregulated on tumor-associated antigen-specific T cells. PD-1, TIM-3, or LAG-3 inhibitors enhance T cell reactivity to tumor antigens and have a synergistic effect. TIM-3+PD-1+CD8+ tumor-infiltrating lymphocytes can inhibit the production of cytokines such as IFN-γ, tumor necrosis factor-α (TNF-α), and IL-2. The combination of TIM-3 blockade and PD-1 blockade may be more effective than TIM-3 or PD-1 blockade alone.
Currently, many clinical trials are looking at TIM-3 as a new way to treat cancer.
Cancer immunotherapy has shown promising therapeutic effects. T cell checkpoint inhibitors are one of the most promising new approaches to treating cancer. TIM-3 can suppress anti-tumor immunity. However, the role of TIM-3 in tumor immunity needs further study. New therapeutic approaches targeting TIM-3 will soon lead to breakthroughs in cancer treatment and improved patient outcomes.
Future directions in TIM-3 research
TIM-3, a checkpoint receptor containing the T cell immunoglobulin and mucin domains, is pivotal in modulating immune responses. Its contribution has been noted in several diseases, including cancer, autoimmunity, and infectious diseases.
There are multiple potential avenues for further TIM-3 research. One of the most promising is the development of novel TIM-3 inhibitors for cancer immunotherapy, with several already in clinical trials and more in development. Another crucial area of interest is the involvement of TIM-3 in T-cell dysfunction and immune exhaustion, particularly in chronic viral infections and cancer. Further research in this area could uncover new therapeutic approaches. Additionally, recent studies have suggested a potential role for TIM-3 in regulating the gut microbiota and modulating intestinal inflammation, offering a promising avenue for developing new therapies for inflammatory bowel disease and other related gut disorders.
In summary, future directions in TIM-3 research include the development of TIM-3 inhibitors for cancer immunotherapy, understanding the role of TIM-3 in immune exhaustion, and investigating the potential of TIM-3 as a target for gut-related disorders.
References
- Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol. 2020 Mar;20(3):173-185. doi: 10.1038/s41577-019-0224-6. Epub 2019 Nov 1. PMID: 31676858; PMCID: PMC7327798.
- Yang DQ, Zhang YM, Wu LY, Wu D, Chang CK. [Research Progress in the Role of Tim3/galectin-9 in Hematological Malignancy–Review]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021 Aug;29(4):1360-1364. Chinese. doi: 10.19746/j.cnki.issn.1009-2137.2021.04.056. PMID: 34362531.
- Herrera-Camacho I, Anaya-Ruiz M, Perez-Santos M, Millán-Pérez Peña L, Bandala C, Landeta G. Cancer immunotherapy using anti-TIM3/PD-1 bispecific antibody: a patent evaluation of EP3356411A1. Expert Opin Ther Pat. 2019 Aug;29(8):587-593. doi: 10.1080/13543776.2019.1637422. Epub 2019 Jul 4. PMID: 31241380.
- Solinas C, De Silva P, Bron D, Willard-Gallo K, Sangiolo D. Significance of TIM3 expression in cancer: From biology to the clinic. Semin Oncol. 2019 Aug-Oct;46(4-5):372-379. doi: 10.1053/j.seminoncol.2019.08.005. Epub 2019 Nov 6. PMID: 31733828.
- Zhao L, Cheng S, Fan L, Zhang B, Xu S. TIM-3: An update on immunotherapy. Int Immunopharmacol. 2021 Oct;99:107933. doi: 10.1016/j.intimp.2021.107933. Epub 2021 Jul 2. PMID: 34224993.
- Friedlaender A, Addeo A, Banna G. New emerging targets in cancer immunotherapy: the role of TIM3. ESMO Open. 2019 Jun 12;4(Suppl 3):e000497. doi: 10.1136/esmoopen-2019-000497. PMID: 31275616; PMCID: PMC6579568.
- Sauer N, Szlasa W, Jonderko L, Oślizło M, Kunachowicz D, Kulbacka J, Karłowicz-Bodalska K. LAG-3 as a Potent Target for Novel Anticancer Therapies of a Wide Range of Tumors. Int J Mol Sci. 2022 Sep 1;23(17):9958. doi: 10.3390/ijms23179958. PMID: 36077354; PMCID: PMC9456311.
- Zhao L, Yu G, Han Q, Cui C, Zhang B. TIM-3: An emerging target in the liver diseases. Scand J Immunol. 2020 Apr;91(4):e12825. doi: 10.1111/sji.12825. Epub 2020 Feb 5. PMID: 31486085.
- Tian T, Li Z. Targeting Tim-3 in Cancer With Resistance to PD-1/PD-L1 Blockade. Front Oncol. 2021 Sep 22;11:731175. doi: 10.3389/fonc.2021.731175. PMID: 34631560; PMCID: PMC8492972.