The Role of Allosteric Inhibitors in Enzyme Regulation and Drug Discovery
Abstract
Allosteric inhibitor is a type of enzyme inhibitor that binds to a site on an enzyme that is different from the enzyme’s active site, known as the allosteric site. This binding event alters the shape of the enzyme, often making it less active or inactive altogether. Allosteric inhibitors can be distinguished from orthosteric inhibitors, which bind directly to the enzyme’s active site, and from competitive inhibitors, which bind the same site as the enzyme’s substrate. Allosteric inhibitors are known to be important in regulating cellular processes, such as enzyme activity, signal transduction, and gene expression. They can be used in drug discovery to target specific allosteric sites on enzymes or proteins, which can provide advantages such as improved selectivity and reduced side effects. In recent years, advances in computational and experimental techniques have enabled the discovery and development of allosteric inhibitors for a range of targets, including kinases, G protein-coupled receptors, and ion channels. Overall, allosteric inhibitors offer a promising avenue for the development of new therapeutics with improved efficacy and safety profiles.
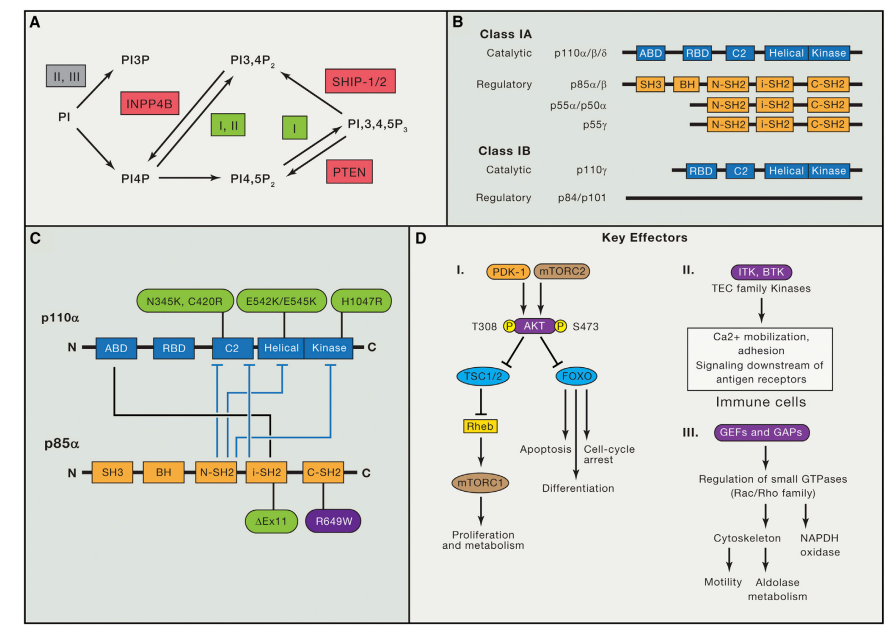
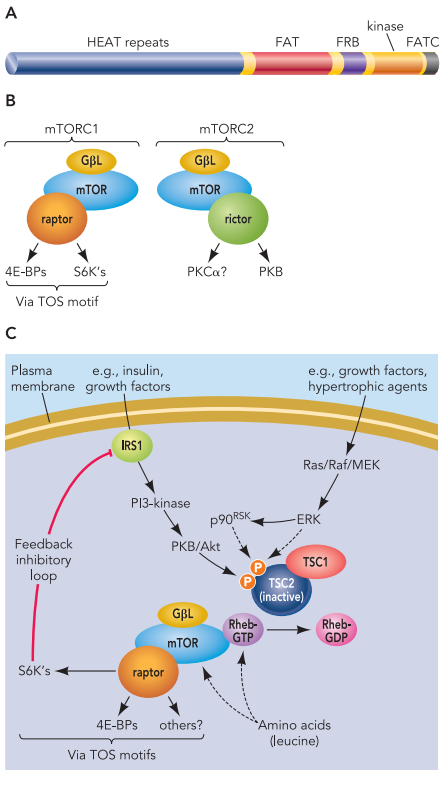
Definition of allosteric regulation in kinases
Allosteric regulation is a type of regulation that occurs when a molecule binds to a protein at a site other than its active site, causing a conformational change that affects the protein’s activity. In kinases, which are enzymes that catalyze the transfer of phosphate groups from ATP to specific substrates, allosteric regulation plays an important role in controlling cellular signaling pathways.
Kinases are typically activated by a variety of extracellular and intracellular signals, including hormones, growth factors, and stress. These signals can induce changes in the kinase’s structure, leading to alterations in its catalytic activity, substrate specificity, and localization. Allosteric regulation of kinases can occur through various mechanisms, including modulation of protein-protein interactions, alteration of kinase domain conformation, and changes in subcellular localization.
Allosteric regulation can have a profound effect on the function and activity of kinases, allowing them to respond dynamically to changes in the cellular environment. For example, allosteric regulation of kinases can be involved in the regulation of cell proliferation, differentiation, and survival. Dysregulation of kinase activity due to mutations or altered allosteric regulation has been implicated in a range of diseases, including cancer, inflammation, and metabolic disorders.
Understanding the molecular mechanisms underlying allosteric regulation of kinases is essential for the development of new therapeutic strategies for these diseases. In recent years, significant progress has been made in the discovery and development of allosteric inhibitors of kinases, which offer the potential for highly selective and potent drug candidates.
Importance of allosteric regulation in drug discovery
Allosteric regulation plays a critical role in drug discovery by offering a unique opportunity to develop highly selective and potent drugs with reduced side effects. Allosteric sites are distinct from active sites, and therefore allosteric modulators can act on a protein without directly interfering with its enzymatic activity. As a result, allosteric modulation can provide advantages over orthosteric inhibitors, which can lead to off-target effects due to their interactions with other proteins that share the same active site.
Moreover, allosteric sites are often more structurally diverse and less conserved than active sites, providing a greater opportunity for developing selective modulators. The structural diversity of allosteric sites also means that they can be targeted by a wider range of chemical scaffolds, offering a larger chemical space for drug discovery.
In addition, allosteric regulation is increasingly recognized as a key mechanism in the regulation of protein function, including enzymes, receptors, and ion channels. Therefore, the development of allosteric modulators can provide a powerful tool for dissecting the molecular mechanisms underlying cellular signaling pathways and identifying new therapeutic targets.
Overall, allosteric regulation is an important aspect of drug discovery that offers a promising avenue for the development of new, more effective and safer drugs.
Allosteric drugs for cancer treatment
Allosteric drugs have shown great promise in cancer treatment, as they can target specific mutations or alterations in the activity of oncogenic kinases, which are often overexpressed or activated in cancer cells. Allosteric inhibitors can bind to sites that are distinct from the ATP-binding pocket and induce conformational changes that prevent kinase activation, leading to reduced tumor growth and cell proliferation.
For example, several allosteric inhibitors have been developed for the treatment of chronic myeloid leukemia (CML), a type of cancer caused by a genetic mutation that leads to overexpression and activation of the BCR-ABL kinase. These inhibitors have been shown to be highly effective in treating CML patients, and have significantly improved the prognosis for this disease.
Allosteric inhibitors have also been developed for the treatment of other types of cancer, such as lung cancer and breast cancer, and are currently being tested in clinical trials. These drugs offer the potential for more targeted and effective cancer treatments with fewer side effects, and are likely to play an increasingly important role in cancer therapy in the future
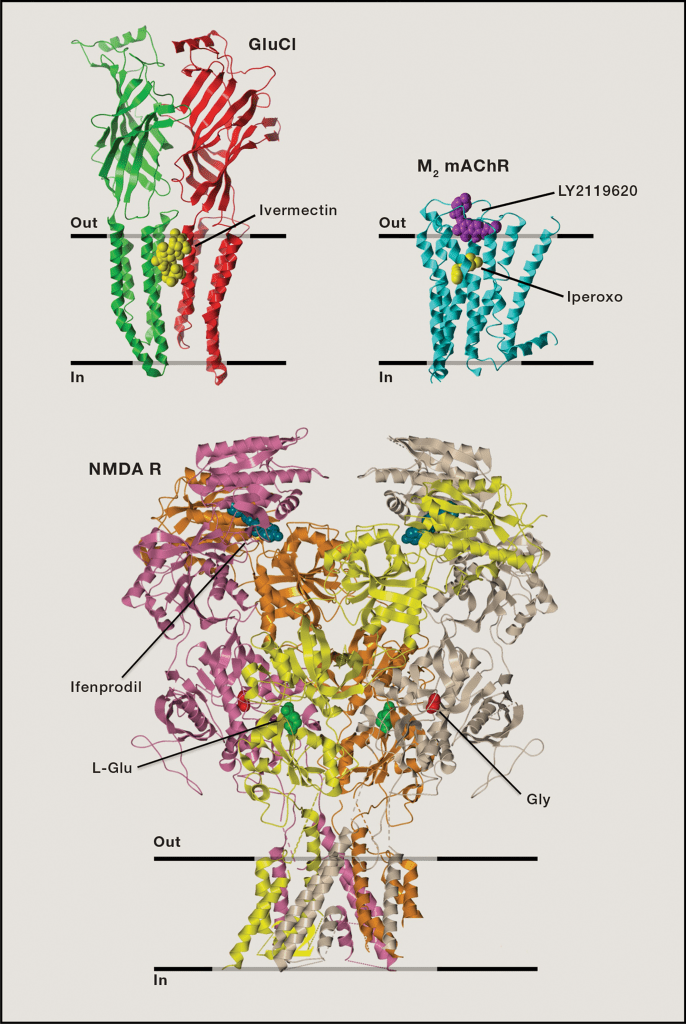
Fig 1 Representative crystal structures of allosteric modulatory sites and their ligands [4].
Shown are the homopentameric Caenorhabditis elegans glutamate-gated chloride channel (GluCl) (PDB: 3RHW), where the allosteric modulator, ivermectin, sits within the transmembrane regions of the channel; the human M2 mAChR (PDB: 4MQT), where the allosteric modulator, LY2119620, sits within the extracellular vestibular entrance to the receptor, above the orthosteric agonist, iperoxo; the rat NMDA R (PDB: 4PE5), where the allosteric modulator, ifenprodil, is located at interfaces between amino terminal domain subunits, whereas the orthosteric co-agonists, L-glutamate and glycine, sit within the ligand binding domain.
The advantages of using allosteric inhibitors
In drug development, inhibitors can target the active site of a protein, known as an orthosteric site, or they can target an allosteric site, which is distinct from the active site. Small molecular inhibitors bind directly to the active site and compete with the substrate for binding, whereas allosteric inhibitors bind to a site elsewhere on the protein, leading to a conformational change that affects the active site.
Allosteric inhibitors have several advantages over small molecular inhibitors. First, they can target proteins that have been difficult to inhibit using small molecular inhibitors. This is because the active site of these proteins may not be well-defined or may be highly conserved across related proteins. Allosteric inhibitors can target sites that are more variable and specific to the target protein, offering a greater degree of selectivity and fewer off-target effects.
Second, allosteric inhibitors can provide greater control over protein activity. By binding to a site that induces a conformational change, allosteric inhibitors can modulate the activity of the protein in a more precise and nuanced way than small molecular inhibitors. This can be particularly useful for proteins that have multiple functions or are involved in complex signaling pathways.
Challenges in developing allosteric inhibitors, including specificity and potency issues
The development of allosteric inhibitors presents several challenges, including issues related to specificity and potency. These challenges arise from the complex nature of allosteric interactions and the need to identify suitable binding sites and ligands.
One of the primary challenges in developing allosteric inhibitors is achieving sufficient selectivity for the target protein. Because allosteric sites may be less well-defined than active sites, there is a risk of off-target effects if the inhibitor binds to similar sites on other proteins. This can be addressed by using computational methods to identify sites that are specific to the target protein, as well as by optimizing the binding properties of the inhibitor through structure-activity relationship studies.
Another challenge is achieving sufficient potency, or binding affinity, of the inhibitor for the allosteric site. Allosteric interactions are often weaker than those at active sites, and the affinity of the inhibitor may be affected by the conformational flexibility of the protein. This can be addressed through the use of fragment-based drug discovery, which focuses on identifying small molecules that bind weakly but specifically to the target site and then building them up into more potent inhibitors.
A related challenge is achieving optimal binding kinetics, as allosteric inhibitors may need to bind and stabilize specific conformations of the protein in order to achieve the desired effect. This can be addressed through the use of kinetic assays to measure the binding and dissociation rates of the inhibitor and optimize its binding kinetics.
Finally, there may be challenges related to the physicochemical properties of the inhibitor, such as its solubility, stability, and cell permeability. These issues can be addressed through the use of chemical modification, such as the addition of solubilizing groups or lipid anchors, as well as through the use of in vitro and in vivo assays to evaluate the properties of the inhibitor.
Despite these challenges, the development of allosteric inhibitors has the potential to provide a powerful approach to drug discovery, with the ability to target a wide range of proteins and provide greater selectivity and control over protein activity. As our understanding of allosteric regulation continues to grow, it is likely that these challenges will be addressed and allosteric inhibitors will become an increasingly important class of drugs.
Examples of successful allosteric kinase inhibitors
Allosteric inhibitors have gained much attention in recent years for their potential to offer better selectivity and potency in targeting specific proteins. Here we discuss specific examples of allosteric inhibitors and their mechanisms of action.
One of the best-known examples is the allosteric inhibitor Imatinib, which is used to treat chronic myeloid leukemia and gastrointestinal stromal tumors. Imatinib targets the Bcr-Abl tyrosine kinase, which is overactive in these cancers. The inhibitor binds to the kinase’s ATP-binding site, but it also induces a conformational change that locks the kinase in an inactive state. This mechanism of action makes it highly selective for the target kinase, as it only inhibits the active form of Bcr-Abl.
Another example of an allosteric inhibitor is the drug Entrectinib, which targets the tyrosine kinase TRK. TRK is implicated in a range of cancers, including lung cancer and pediatric tumors. Entrectinib binds to an allosteric site on TRK, which is separate from the kinase’s active site. This binding causes a conformational change that prevents ATP from binding to the kinase and inhibits its activity.
Another promising example of allosteric inhibitors is the drug Sotorasib, which targets KRAS G12C, a mutation commonly found in lung cancer. KRAS is a notoriously difficult target, but Sotorasib binds to an allosteric site on the protein, causing a conformational change that locks it in an inactive state. This mechanism of action makes it highly specific for the mutant form of KRAS and spares the wild-type protein.
Overall, allosteric inhibitors have shown great promise in drug development due to their potential for high selectivity and potency. The mechanism of action of allosteric inhibitors is different from that of orthosteric inhibitors, which target the active site of the protein. However, developing allosteric inhibitors can be challenging due to the need to identify specific allosteric sites on the protein and to ensure that the inhibitor binds selectively to those sites. Nevertheless, with continued advances in technology and computational methods, it is likely that many more allosteric inhibitors will be developed for a wide range of diseases.
Allosteric inhibitors’ potential as drug candidates
All of the above-described allosteric inhibitors have demonstrated potential as drug candidates due to their specificity, potency, and unique mechanisms of action. For instance, the allosteric inhibitors targeting the BCR-ABL fusion protein, such as GNF-2, allosterically inhibit the oncogenic protein by binding to its myristoyl binding pocket, thereby preventing its autoinhibition and leading to apoptosis of cancer cells. These inhibitors have shown promising preclinical results in animal models of chronic myeloid leukemia (CML) and are currently undergoing clinical trials.
Similarly, the allosteric inhibitors targeting RAF kinases, such as PLX4032 and dabrafenib, have demonstrated efficacy in the treatment of BRAF-mutant melanoma. These inhibitors bind to the allosteric pocket of the kinase domain, thereby stabilizing the inactive conformation of the protein and inhibiting its activation. The clinical success of these inhibitors has led to the development of second-generation inhibitors, such as vemurafenib and encorafenib, which have improved pharmacokinetic properties and potency.
Moreover, the allosteric inhibitors targeting the estrogen receptor (ER), such as fulvestrant, have demonstrated efficacy in the treatment of ER-positive breast cancer. Fulvestrant binds to the ER and induces a conformational change that leads to the degradation of the receptor, thereby preventing its activity. This mechanism of action is unique compared to other ER-targeting drugs, such as tamoxifen and aromatase inhibitors, which only inhibit the activity of the receptor.
Overall, the development of allosteric inhibitors has shown promise in addressing the limitations of orthosteric inhibitors and has led to the discovery of novel drug targets and mechanisms of action. While there are still challenges in developing allosteric inhibitors, such as specificity and potency issues, the potential benefits of these inhibitors make them an attractive area of drug development.
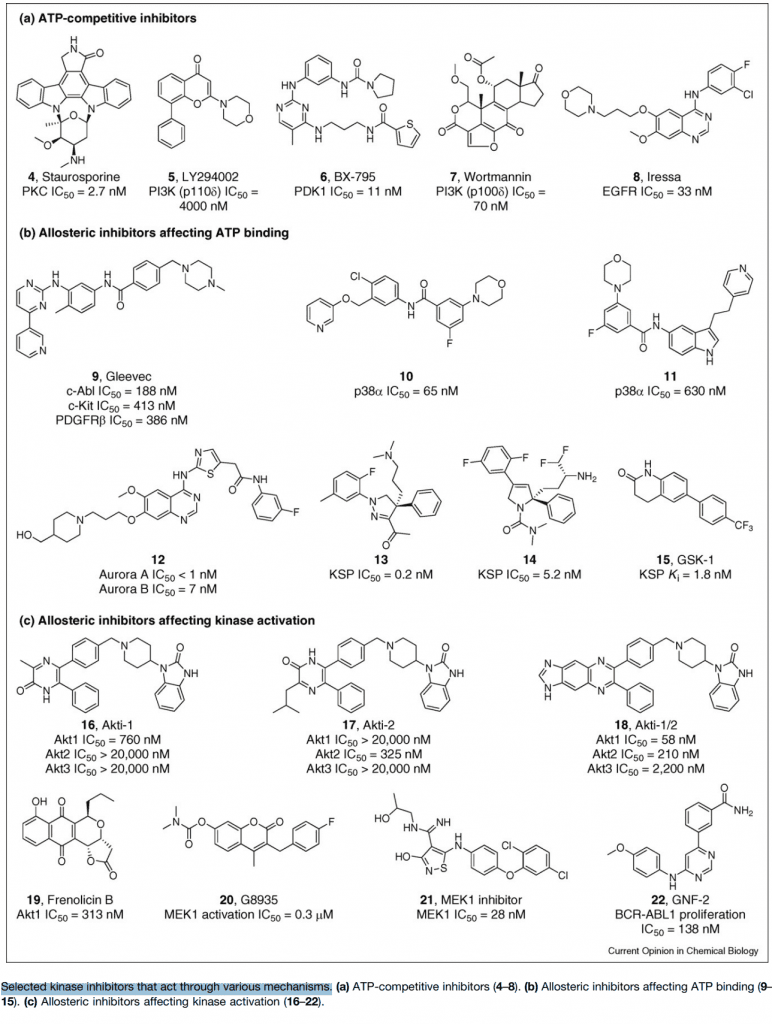
Fig 2. Selected kinase inhibitors that act through various mechanisms.[5] (a) ATP-competitive inhibitors (4–8). (b) Allosteric inhibitors affecting ATP binding (9–15). (c) Allosteric inhibitors affecting kinase activation (16–22).
Potential of allosteric inhibitors in drug discovery
Allosteric inhibitors have emerged as a promising class of therapeutics in drug discovery, with great potential for targeting a wide range of diseases. The ability of allosteric inhibitors to selectively target specific conformations of proteins, as opposed to the more conserved active sites targeted by traditional orthosteric inhibitors, makes them an attractive option for drug discovery. Additionally, allosteric inhibitors can often achieve higher potency and specificity than orthosteric inhibitors, as well as overcome drug resistance mechanisms.
One potential application of allosteric inhibitors is in the treatment of cancer. Many cancer-associated mutations affect the allosteric regulation of proteins, leading to dysregulated cell signaling and proliferation. Allosteric inhibitors that target these mutant proteins have shown promise in preclinical and clinical trials, such as the BTK inhibitor acalabrutinib for the treatment of B-cell malignancies and the EGFR inhibitor osimertinib for non-small cell lung cancer.
Allosteric inhibitors have also shown potential for treating other diseases, such as Alzheimer’s disease, where they can target specific conformations of proteins involved in the disease pathology. For example, the gamma-secretase inhibitor avagacestat targets the allosteric site of the gamma-secretase enzyme, which is involved in the production of beta-amyloid peptides that contribute to Alzheimer’s disease.
The use of computational methods has greatly facilitated the discovery of allosteric inhibitors. Virtual screening and molecular dynamics simulations can be used to identify potential allosteric sites and predict the binding of small molecules to these sites. Additionally, fragment-based drug design and covalent inhibitors can be used to design specific and potent allosteric inhibitors.
Despite their potential, the development of allosteric inhibitors still faces challenges. One challenge is the identification of specific and druggable allosteric sites, as well as the optimization of compounds to achieve optimal potency, selectivity, and pharmacokinetic properties. Additionally, off-target effects and toxicity can be a concern, as allosteric sites may be present in multiple proteins.
In conclusion, allosteric inhibitors represent a promising and rapidly growing class of therapeutics with great potential for treating a wide range of diseases. The use of computational methods, as well as advances in understanding the allosteric regulation of proteins, has greatly facilitated the discovery of allosteric inhibitors. However, the development of allosteric inhibitors still faces challenges, and further research and development are needed to fully realize their potential as effective therapeutics.
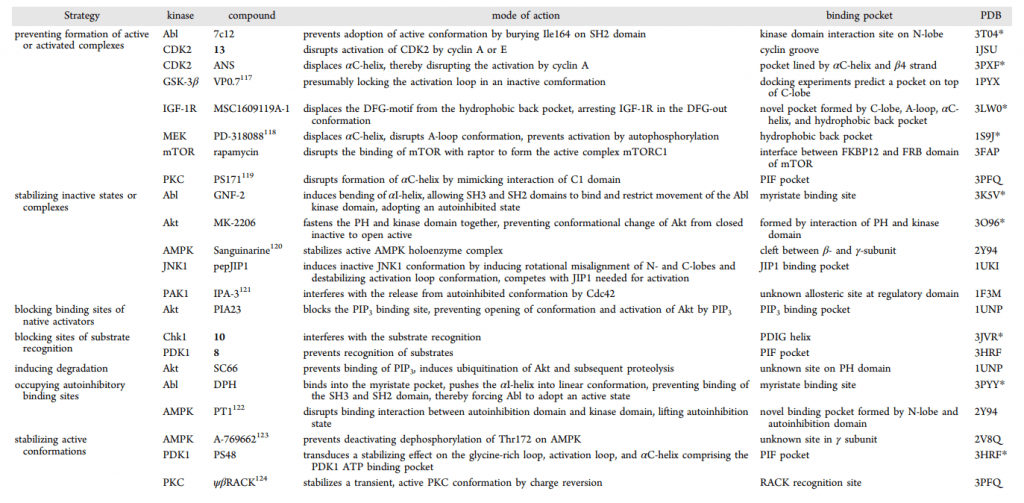
Table 1. Overview of Representative Allosteric Kinase Modulators [6], Their Mode and Site of Action, and the PDB Entry Codes of the Respective X-ray Structures of Its Target Kinase; PDB Codes That Have an Asterisk (*) Show the Actual Allosteric Modulator Bound
Emerging trends and technologies for allosteric inhibitor development
Recent advancements in technology have led to the development of new tools and methods for identifying and designing allosteric inhibitors with improved efficacy, specificity, and potency. Here are some of the emerging trends and technologies in allosteric inhibitor development:
Fragment-based drug discovery: Fragment-based drug discovery (FBDD) is a powerful approach for identifying novel allosteric inhibitors. FBDD involves screening small fragments that bind to a target protein and then building up the fragments into larger compounds. This approach can lead to the identification of highly specific and potent inhibitors.
Structure-based drug design: Structure-based drug design (SBDD) is another powerful approach for designing allosteric inhibitors. SBDD involves using the three-dimensional structure of a target protein to design compounds that bind to specific sites on the protein. This approach can be used to optimize the binding affinity, specificity, and selectivity of allosteric inhibitors.
Computational methods: Computational methods, such as molecular dynamics simulations, free energy calculations, and virtual screening, are increasingly being used to identify and optimize allosteric inhibitors. These methods can help to predict the binding affinity and selectivity of compounds, as well as their ability to modulate protein function.
Covalent allosteric inhibitors: Covalent allosteric inhibitors are a new class of compounds that covalently modify allosteric sites on a target protein, leading to prolonged inhibition. These inhibitors have the potential to overcome issues related to potency and selectivity that are commonly associated with non-covalent inhibitors.
PROTACs: Proteolysis-targeting chimeras (PROTACs) are a new class of compounds that can induce targeted protein degradation. PROTACs consist of a ligand that binds to an allosteric site on a target protein and a ligand that binds to an E3 ubiquitin ligase. This results in the ubiquitination and subsequent degradation of the target protein, leading to a more complete and sustained inhibition.
In conclusion, the emerging trends and technologies in allosteric inhibitor development offer exciting opportunities for drug discovery. With the development of more specific, potent, and efficacious allosteric inhibitors, it is expected that this class of drugs will continue to play an increasingly important role in the treatment of various diseases.
Reference
- Wu, P., Clausen, M. H., & Nielsen, T. E. (2015). Allosteric small-molecule kinase inhibitors. Pharmacology & therapeutics, 156, 59-68.
- Nussinov, R., Zhang, M., Maloney, R., Liu, Y., Tsai, C. J., & Jang, H. (2022). Allostery: allosteric cancer drivers and innovative allosteric drugs. Journal of molecular biology, 167569.
- Daura, X. (2019). Advances in the computational identification of allosteric sites and pathways in proteins. Protein Allostery in Drug Discovery, 141-169.
- Changeux, J. P., & Christopoulos, A. (2017). Allosteric modulation as a unifying mechanism for receptor function and regulation. Diabetes, obesity and metabolism, 19, 4-21.
- Lewis, J. A., Lebois, E. P., & Lindsley, C. W. (2008). Allosteric modulation of kinases and GPCRs: design principles and structural diversity. Current opinion in chemical biology, 12(3), 269-280.
- Fang, Z., Grütter, C., & Rauh, D. (2013). Strategies for the selective regulation of kinases with allosteric modulators: exploiting exclusive structural features. ACS chemical biology, 8(1), 58-70.
- Sheik Amamuddy, O., Veldman, W., Manyumwa, C., Khairallah, A., Agajanian, S., Oluyemi, O., … & Tastan Bishop, Ö. (2020). Integrated computational approaches and tools for allosteric drug discovery. International journal of molecular sciences, 21(3), 847.
- Abdeldayem, A., Raouf, Y. S., Constantinescu, S. N., Moriggl, R., & Gunning, P. T. (2020). Advances in covalent kinase inhibitors. Chemical Society Reviews, 49(9), 2617-2687.