The Renin-Angiotensin System Revisited: Dual Pathways, Tissue-Specific Roles, and Emerging Therapeutic Opportunities
Abstract
The renin-angiotensin system (RAS) is a multifaceted hormonal network that plays a pivotal role in cardiovascular, renal, metabolic, and neurological regulation. Traditionally viewed as a circulating endocrine system comprising renin, angiotensin II (Ang II), and aldosterone, RAS is now recognized to include local or tissue-specific systems (tRAS) with autocrine and paracrine functions. Beyond the classical ACE–Ang II–AT1 receptor pathway, which promotes vasoconstriction, fibrosis, and inflammation, the discovery of the counter-regulatory arm—centered on ACE2, Ang-(1-7), Mas receptor, and alamandine–MrgD receptor—has revealed mechanisms that oppose these deleterious effects through vasodilation, anti-inflammatory activity, and metabolic benefits. Dysregulation of the balance between these two pathways contributes to the pathogenesis of hypertension, heart failure, chronic kidney disease, obesity, and neurodegenerative disorders. Current therapies, such as ACE inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonists, primarily target the classical pathway and have improved clinical outcomes. Future strategies aim to enhance protective pathway activity through ACE2 activators, peptide analogs, receptor agonists, and chymase inhibitors, potentially offering dual-action interventions. This updated understanding of systemic and tissue RAS opens avenues for precision-targeted therapeutics that integrate cardiovascular, metabolic, and organ-specific protection.
Renin-Angiotensin System: Updated Overview
The renin-angiotensin system (RAS) is a complex hormonal network central to maintaining blood volume, electrolyte balance, and vascular resistance. Traditionally, RAS was understood as a circulating endocrine system involving three main components—renin, angiotensin II (Ang II), and aldosterone—working in sequence to regulate salt and water homeostasis and blood pressure. This classical perspective emphasized the systemic, hormone-mediated control of cardiovascular and renal physiology.
Recent discoveries, however, have transformed our understanding of RAS. It is now recognized as ubiquitous, with both systemic and local or tissue RAS (tRAS) present in organs such as the heart, kidneys, brain, lungs, blood vessels, adrenal glands, adipose tissue, ovaries, testes, and skin. In these tissues, RAS operates through paracrine and autocrine mechanisms, influencing local cellular processes beyond systemic circulation. Local RAS activity is implicated in diverse physiological roles, including tissue growth, repair, and metabolic regulation.
A major conceptual shift has been the identification of a counter-regulatory or protective pathway that opposes the classical pathway’s vasoconstrictive, pro-fibrotic, and pro-inflammatory actions. This pathway involves enzymes such as angiotensin-converting enzyme 2 (ACE2) and bioactive peptides like angiotensin-(1-7) and alamandine, which act through the Mas receptor (MasR) and Mas-related G-protein coupled receptor D (MrgD) to promote vasodilation, anti-fibrotic activity, anti-inflammation, and metabolic benefits. The balance between the classical and counter-regulatory pathways is critical to cardiovascular and metabolic health.
The RAS is implicated in a wide range of pathophysiological conditions, including hypertension, heart failure, kidney disease, obesity, insulin resistance, and neurological disorders. Overactivation of the classical pathway contributes to vascular dysfunction, oxidative stress, and fibrosis, while underactivation of the protective pathway may diminish the body’s capacity to counteract these harmful effects.
This evolving understanding of RAS has significant therapeutic implications. Current pharmacological strategies—such as angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), and mineralocorticoid receptor antagonists (MRA)—primarily target the classical pathway to reduce its pathological influence. Meanwhile, new research is exploring approaches to enhance the protective arm of RAS, potentially offering additional benefits in controlling blood pressure, protecting organ function, and improving metabolic outcomes.
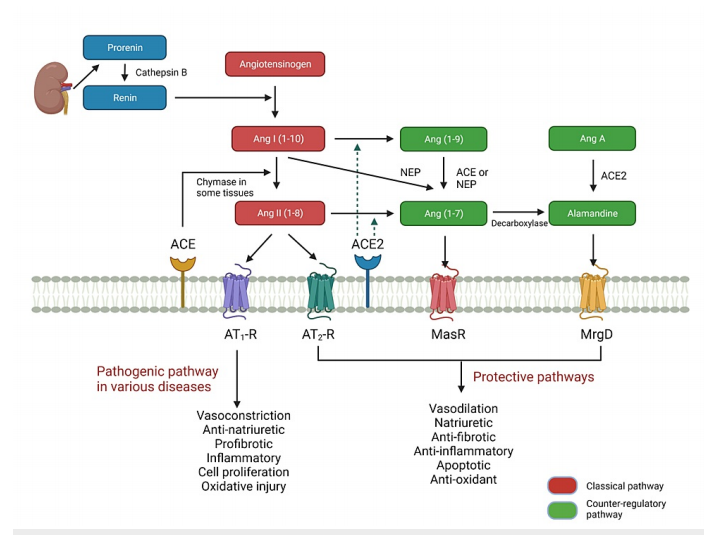
FIGURE 1: Classical and counter-regulatory pathways in the reninangiotensin system.
In summary, the RAS is no longer viewed solely as a simple hormonal cascade for circulatory control. It is now recognized as a multifaceted, tissue-specific system with dual arms—classical and counter-regulatory—whose dynamic interplay influences cardiovascular, renal, metabolic, and neurological health.
Classical Pathway of the Renin-Angiotensin System
The classical pathway of the renin-angiotensin system (RAS) begins with the production and activation of renin, an enzyme secreted by juxtaglomerular cells in the kidney. Renin release is stimulated by reduced renal perfusion, low sodium chloride delivery to the distal tubule, sympathetic activation via β1-adrenergic receptors, and certain humoral factors. Prorenin, an inactive precursor of renin, is also constitutively secreted in various tissues, with its role in circulation still under investigation.
Renin cleaves angiotensinogen, a glycoprotein primarily produced in the liver, into angiotensin I (Ang I), an inactive decapeptide. Ang I is then converted into angiotensin II (Ang II) by angiotensin-converting enzyme 1 (ACE1), which is abundant on the endothelial surface, especially in the pulmonary circulation.
Ang II is the principal effector peptide of the classical RAS, exerting its effects through two receptor subtypes: AT1-R and AT2-R. The AT1-R mediates most of the classical actions—vasoconstriction, sodium retention via proximal tubular reabsorption, stimulation of aldosterone secretion from the adrenal cortex, enhancement of sympathetic activity, and promotion of vasopressin release. Pathologically, AT1-R activation contributes to oxidative stress, fibrosis, endothelial dysfunction, and hypertrophy, which are implicated in hypertension, heart failure, atherosclerosis, and kidney disease.
Aldosterone, synthesized in the zona glomerulosa of the adrenal cortex, acts mainly on the kidneys to promote sodium and water reabsorption and potassium excretion, but it also has non-epithelial effects on cardiovascular and central nervous systems, influencing vascular tone and remodeling.
The classical pathway’s overactivation can lead to chronic cardiovascular and renal pathologies, making it a key therapeutic target for antihypertensive drugs such as ACE inhibitors, angiotensin receptor blockers (ARBs), and mineralocorticoid receptor antagonists.
Counter-Regulatory (Protective) Pathway of the Renin-Angiotensin System
The counter-regulatory or protective pathway of the renin-angiotensin system (RAS) provides a physiological balance to the classical pathway’s vasoconstrictive and pro-fibrotic effects. Central to this protective arm is angiotensin-converting enzyme 2 (ACE2), a monocarboxypeptidase structurally related to ACE1 but with distinct enzymatic activity. ACE2 converts angiotensin II (Ang II) into angiotensin-(1-7) [Ang-(1-7)] and angiotensin I (Ang I) into angiotensin-(1-9). This enzymatic shift reduces Ang II availability while generating peptides with vasodilatory, anti-inflammatory, and anti-fibrotic properties.
Ang-(1-7) is the principal bioactive peptide of the protective pathway. It acts mainly through the Mas receptor (MasR) and, to some extent, the Mas-related G-protein coupled receptor D (MrgD). Ang-(1-7) opposes Ang II–AT1-R–mediated actions by promoting nitric oxide production, enhancing endothelial function, inhibiting fibrosis, and improving lipid and glucose metabolism. Experimental models have demonstrated its role in preventing cardiac remodeling after myocardial infarction and attenuating hypertension-induced organ damage.
Angiotensin-(1-9), another ACE2-derived peptide, interacts with the AT2 receptor (AT2-R) to exert anti-hypertrophic, vasodilatory, and anti-inflammatory effects, contributing to cardiovascular and renal protection.
Alamandine, a heptapeptide structurally related to Ang-(1-7), is produced either from decarboxylation of Ang-(1-7) or conversion of Ang A by ACE2. Alamandine signals predominantly via the MrgD receptor to induce vasodilation, reduce oxidative stress, and attenuate fibrosis in cardiac and renal tissues.
The protective pathway’s influence extends to metabolic regulation. Activation of the ACE2/Ang-(1-7)/MasR axis has been linked to increased brown adipose tissue activity, browning of white adipose tissue, and improved insulin sensitivity, offering potential therapeutic applications in obesity and metabolic syndrome.
Pathologically, reduced ACE2 expression—such as that observed in certain cardiovascular diseases or after Ang II–mediated ADAM17 activation—can shift the RAS balance toward the harmful classical pathway. Enhancing the protective pathway through ACE2 activators, Ang-(1-7) analogs, MasR agonists, or alamandine-based therapies represents an emerging area of cardiovascular, renal, and metabolic drug development.
Tissue-Specific Renin-Angiotensin System (tRAS)
The tissue-specific renin-angiotensin system (tRAS) refers to the local synthesis and activity of RAS components within individual organs and tissues, functioning alongside or independently of the circulating RAS. These local systems produce critical elements of both the classical and counter-regulatory pathways, influencing physiological and pathological processes through autocrine, paracrine, and intracrine signaling.
Cardiac tRAS plays a central role in regulating structural and functional responses to stimuli such as ischemia, pressure overload, and hormonal stress. Angiotensin II (Ang II) is generated locally via ACE1 and ACE-independent enzymes like chymase. While AT1 receptor activation promotes fibrosis, hypertrophy, and remodeling, ACE2 and Ang-(1-7) within cardiac tissues counteract these effects through anti-fibrotic, anti-inflammatory, and vasodilatory mechanisms. Animal models have shown that genetic deletion of the MrgD receptor in the heart leads to cardiomyopathy, highlighting the protective role of alamandine signaling.
Adipose tissue tRAS regulates fat development, distribution, and metabolism. In white adipose tissue (WAT), overactivation of the classical pathway contributes to obesity, insulin resistance, and hypertension, partly through increased angiotensinogen expression in visceral fat. In contrast, activation of the ACE2/Ang-(1-7)/MasR axis promotes browning of WAT, enhances brown adipose tissue (BAT) activity, and improves glucose uptake and insulin sensitivity—effects with therapeutic potential for metabolic disorders.
Brain tRAS influences blood pressure control, water balance, neuroendocrine regulation, and stress responses. Locally generated Ang II interacts with AT1 and AT2 receptors to regulate vascular tone and sympathetic output. The protective components—ACE2, Ang-(1-7), MasR, and MrgD—modulate neuroprotection, reduce oxidative stress, and may have roles in mitigating neurodegenerative processes. Dysregulation of brain tRAS has been linked to hypertension, stroke, Alzheimer’s disease, and Parkinson’s disease.
Other tissues—including the kidneys, lungs, reproductive organs, and skin—also exhibit distinct RAS activity profiles, often adapting to local physiological demands or pathological insults. Because tRAS can contribute to organ-specific damage or protection, targeting local RAS components represents an important therapeutic frontier, offering the possibility of tissue-selective interventions to manage cardiovascular, metabolic, and neurological diseases.
Therapeutic Implications of the Renin-Angiotensin System
Therapeutic strategies targeting the renin-angiotensin system (RAS) have evolved considerably as our understanding of its dual pathways—classical and counter-regulatory—has expanded. Most current treatments aim to block the classical ACE–Ang II–AT1 receptor axis, which drives vasoconstriction, sodium retention, fibrosis, inflammation, and oxidative stress in cardiovascular, renal, and metabolic diseases.
Classical pathway blockade remains a cornerstone of hypertension and heart failure management. Angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs) not only lower blood pressure but also improve cardiovascular outcomes by reducing hospitalizations for heart failure, lowering cardiovascular mortality, and slowing the progression of chronic kidney disease (CKD). These agents can also enhance insulin sensitivity and modestly reduce the incidence of type 2 diabetes. Mineralocorticoid receptor antagonists (MRA), such as spironolactone, eplerenone, and the newer non-steroidal finerenone, improve survival in heart failure and provide renal protection in diabetic kidney disease. More recently, aldosterone synthase inhibitors like baxdrostat have shown promise in resistant hypertension.
Emerging therapeutic directions focus on enhancing the counter-regulatory (protective) pathway to restore RAS balance. This includes pharmacological activation of the ACE2–Ang-(1-7)–Mas receptor axis and the alamandine–MrgD receptor axis, which promote vasodilation, anti-inflammatory effects, anti-fibrosis, and metabolic benefits. Potential interventions include ACE2 activators, Ang-(1-7) analogs, MasR agonists, alamandine analogs, and MrgD receptor agonists. These agents could complement classical pathway blockers, offering combined inhibition of harmful effects and stimulation of protective mechanisms.
From a metabolic perspective, counter-regulatory pathway stimulation has been associated with increased brown adipose tissue activity, browning of white adipose tissue, and improved glucose metabolism in experimental models. This opens possibilities for addressing obesity, metabolic syndrome, and type 2 diabetes through RAS modulation.
Chymase inhibitors represent another promising class, targeting Ang II production through ACE-independent pathways, especially in tissues like the heart where chymase activity increases with age and disease.
Ultimately, future therapeutic strategies may integrate dual-action drugs—capable of simultaneously blocking the classical pathway while activating the protective pathway—to provide broader cardiovascular, renal, and metabolic protection. This precision approach could allow tissue-specific targeting of RAS, minimizing systemic side effects while maximizing organ protection.
In summary, the RAS offers multiple intervention points, and the shift from exclusive inhibition to combined modulation reflects a new era in cardiovascular, renal, and metabolic therapeutics.
Conclusions
Advances in research over the past two decades have transformed the understanding of the renin-angiotensin system (RAS) from a simple hormonal cascade regulating blood pressure and fluid balance to a multifaceted, tissue-integrated network with dual functional arms. The classical pathway—mediated by the ACE–Ang II–AT1 receptor axis—remains central to circulatory control but is also implicated in pathological processes such as vascular inflammation, fibrosis, oxidative stress, and metabolic dysfunction.
In parallel, the discovery of the counter-regulatory (protective) pathway, centered on the ACE2–Ang-(1-7)–Mas receptor and alamandine–MrgD receptor axes, has revealed a physiological system that opposes the deleterious effects of the classical arm. This pathway exerts vasodilatory, anti-fibrotic, anti-inflammatory, and metabolic benefits, offering a natural balancing mechanism within the RAS.
Importantly, the RAS is not limited to systemic circulation. Local or tissue RAS (tRAS) is now recognized in multiple organs, including the heart, kidneys, brain, and adipose tissue, where it regulates specific physiological processes and contributes to disease pathology. Dysregulation of local RAS components can exacerbate cardiovascular, renal, metabolic, and neurological disorders.
From a therapeutic standpoint, existing interventions—ACE inhibitors, ARBs, and MRAs—primarily target the classical pathway and have yielded significant improvements in cardiovascular and renal outcomes. However, these approaches address only one side of the system. Future therapeutic strategies are shifting toward dual modulation—simultaneously blocking harmful classical signaling while enhancing protective counter-regulatory activity. Potential innovations include ACE2 activators, Ang-(1-7) analogs, MasR agonists, alamandine derivatives, and chymase inhibitors, as well as tissue-specific delivery systems to maximize efficacy and reduce systemic side effects.
Overall, the evolving model of the RAS underscores its complex interplay between systemic and tissue-specific components and between its harmful and protective arms. Understanding this balance not only deepens insight into disease mechanisms but also opens opportunities for more targeted, effective therapies. As research continues, integrated RAS modulation could become a cornerstone in managing hypertension, heart failure, kidney disease, obesity, and metabolic syndrome.
References
Kanugula, A. K., Kaur, J., Batra, J., Ankireddypalli, A. R., & Velagapudi, R. (2023). Renin-angiotensin system: Updated understanding and role in physiological and pathophysiological states. Cureus, 15(6), e40725.
https://doi.org/10.7759/cureus.40725
Paul, M., Poyan Mehr, A., & Kreutz, R. (2006). Physiology of local renin-angiotensin systems. Physiological Reviews, 86(3), 747–803.
https://doi.org/10.1152/physrev.00036.2005
Santos, R. A. S., Oudit, G. Y., Verano-Braga, T., Canta, G., Steckelings, U. M., & Bader, M. (2019). The renin-angiotensin system: Going beyond the classical paradigms. American Journal of Physiology-Heart and Circulatory Physiology, 316(5), H958–H970.
https://doi.org/10.1152/ajpheart.00723.2018
Wu, C. H., Mohammadmoradi, S., Chen, J. Z., Sawada, H., Daugherty, A., & Lu, H. S. (2018). Renin-angiotensin system and cardiovascular functions. Arteriosclerosis, Thrombosis, and Vascular Biology, 38(6), e108–e116.
https://doi.org/10.1161/ATVBAHA.118.311282
Karnik, S. S., Unal, H., Kemp, J. R., et al. (2015). Angiotensin receptors: Interpreters of pathophysiological angiotensinergic stimuli. Pharmacological Reviews, 67(4), 754–819.
https://doi.org/10.1124/pr.114.010454
Mehta, P. K., & Griendling, K. K. (2007). Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. American Journal of Physiology-Cell Physiology, 292(1), C82–C97.
https://doi.org/10.1152/ajpcell.00287.2006
Williams, G. H. (2005). Aldosterone biosynthesis, regulation, and classical mechanism of action. Heart Failure Reviews, 10(1), 7–13.
https://doi.org/10.1007/s10741-005-2343-3
Santos, R. A. S., Sampaio, W. O., Alzamora, A. C., et al. (2018). The ACE2/angiotensin-(1-7)/Mas axis of the renin-angiotensin system: Focus on angiotensin-(1-7). Physiological Reviews, 98(1), 505–553.
https://doi.org/10.1152/physrev.00023.2016
Patel, V. B., Zhong, J. C., Grant, M. B., & Oudit, G. Y. (2016). Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circulation Research, 118(8), 1313–1326.
https://doi.org/10.1161/CIRCRESAHA.116.307708
Lautner, R. Q., Villela, D. C., Fraga-Silva, R. A., et al. (2013). Discovery and characterization of alamandine: A novel component of the renin-angiotensin system. Circulation Research, 112(8), 1104–1111.
https://doi.org/10.1161/CIRCRESAHA.113.301077
Nehme, A., Zouein, F. A., Zayeri, Z. D., & Zibara, K. (2019). An update on the tissue renin angiotensin system and its role in physiology and pathology. Journal of Cardiovascular Development and Disease, 6(2), 14.