The Jasmonate Journey: How Plants Make, Move, and Manage Their Stress Hormones
Abstract
Jasmonates are a vital class of plant hormones that regulate a wide range of physiological processes, from stress responses and immunity to growth and reproduction. Recent research has revealed that the effectiveness of jasmonates depends not only on their biosynthesis and perception but also on their precise intracellular and intercellular transport. This blog explores the multi-compartmental pathway of jasmonate metabolism, highlights the discovery and function of key jasmonate transporters, and explains how jasmonate signaling triggers gene expression in response to environmental stimuli. It also examines the implications of jasmonate transport for crop improvement and plant evolutionary biology. Understanding the full jasmonate pathway—from synthesis to signaling—offers new strategies for enhancing plant resilience and productivity.
Introduction: What Are Jasmonates and Why They Matter
Plants, although rooted in place, have evolved highly dynamic ways to respond to environmental threats and internal developmental cues. One of the most critical tools in their arsenal is a class of plant hormones called jasmonates (JAs). These lipid-derived signaling molecules play a central role in orchestrating responses to wounding, herbivore attack, and pathogen invasion, as well as regulating growth, reproduction, and metabolic homeostasis.
The most well-known and bioactive member of this hormone family is jasmonoyl-isoleucine (JA-Ile). It functions as a potent molecular signal that activates gene expression in response to stress and developmental triggers. Through precise intracellular localization and receptor-mediated signaling, JA-Ile enables plants to finely balance their defense and growth—a trade-off crucial for survival in ever-changing environments.
Jasmonates are not just passive messengers; they are deeply embedded in complex networks of biosynthesis, metabolism, transport, and signal transduction. The biosynthesis of jasmonic acid begins in the chloroplast and continues through the peroxisome and cytosol, leading to the formation of JA-Ile. This molecule is then recognized by a nuclear receptor complex (SCF^COI1-JAZ), triggering a cascade of transcriptional changes.
Recent research, including the 2021 review by Li et al., emphasizes that jasmonate function is not limited to their production and perception. Instead, their spatial distribution within the cell—controlled by specific transporters—is critical to understanding how plants coordinate local and systemic responses. Transport mechanisms allow JA precursors, like OPDA, and active forms, like JA-Ile, to reach precise cellular locations, ensuring timely and targeted responses.
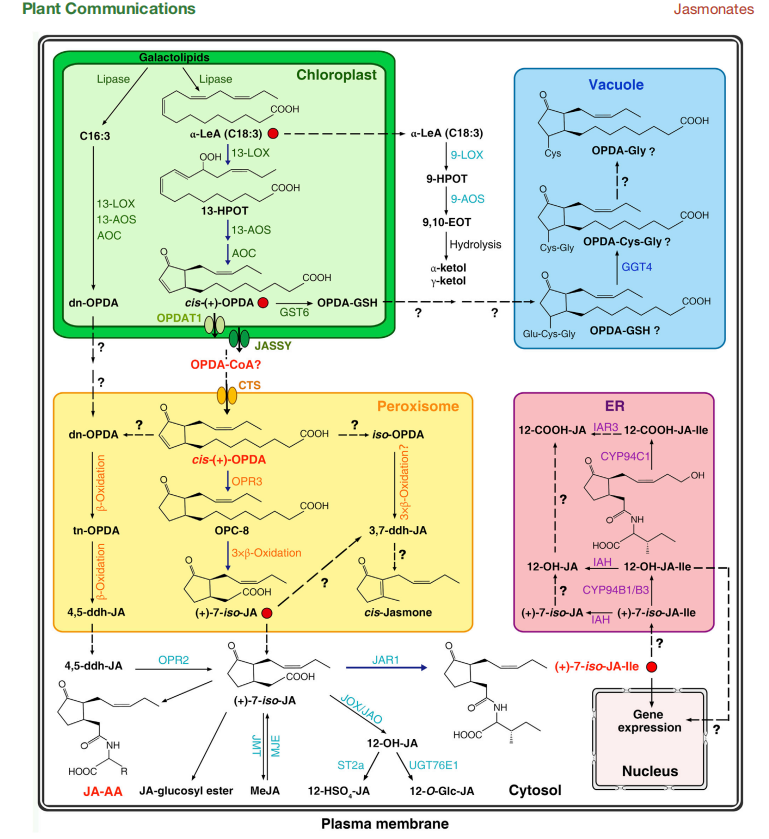
Figure 1. Intracellular compartmentation of the biosynthesis, metabolism, and signaling of JAs.
In essence, jasmonates are master regulators of plant resilience. Their study offers key insights into improving crop tolerance to stresses and designing innovative strategies in plant biotechnology and sustainable agriculture.
Inside the Cell: Where Jasmonates Are Made and Modified
The biological effectiveness of jasmonates (JAs) is rooted not only in their signaling capabilities but also in their complex biosynthesis and intracellular trafficking. The synthesis of jasmonic acid (JA), the precursor to the bioactive jasmonoyl-isoleucine (JA-Ile), is a multi-step process distributed across several cellular compartments—each playing a pivotal role in modulating the hormonal response.
The journey begins in the chloroplast, where polyunsaturated fatty acids like α-linolenic acid (α-LeA) are oxygenated by lipoxygenases (LOX), producing 13-hydroperoxy-octadecatrienoic acid (13-HPOT). This compound is transformed by allene oxide synthase (AOS) and allene oxide cyclase (AOC) into 12-oxo-phytodienoic acid (OPDA), a key jasmonate precursor. OPDA must then exit the chloroplast, facilitated by specialized transport proteins such as JASSY, and enter the peroxisome, where it is converted into JA through reduction and β-oxidation cycles.
Within the cytosol, JA is modified by JAR1, an enzyme that conjugates JA with isoleucine to form JA-Ile—the active form required for initiating gene expression. This conjugation represents a critical control point, linking metabolic flux with environmental signals. However, JA doesn’t stop there. It continues to undergo hydroxylation, methylation, glycosylation, and sulfation, producing a range of derivatives with varying biological activity.
The vacuole and endoplasmic reticulum (ER) serve as storage and detoxification sites, particularly for excess OPDA and JA-Ile. The ER is also the site of JA-Ile catabolism, primarily through the action of cytochrome P450 enzymes (CYP94s), which convert JA-Ile into less active or inactive forms like 12-OH-JA-Ile.
This compartmentalization is far from arbitrary—it allows plants to regulate hormone availability with temporal and spatial precision, directing responses only when and where they are needed. As Li et al. emphasize, understanding this intracellular orchestration is essential to fully grasp the versatility and efficiency of jasmonate signaling.
Transport Is Everything: Moving Jasmonates to the Right Place
While the biosynthesis and signaling of jasmonates (JAs) have been widely studied, their transport across cellular membranes is emerging as a critical layer of regulation. Far from being static molecules, JAs are actively moved between organelles, tissues, and even from leaf to leaf, enabling plants to mount coordinated responses to environmental stressors and developmental signals.
At the heart of this system are ATP-binding cassette (ABC) transporters, particularly the jasmonate transporter (JAT) family, which facilitate the intracellular and intercellular movement of jasmonates. One of the best-characterized members, AtJAT1 (also known as AtABCG16), is dually localized on the plasma membrane (PM) and the nuclear envelope (NE). It performs two key functions: exporting JA out of the cytosol and importing JA-Ile into the nucleus, where it initiates gene transcription via the COI1-JAZ receptor complex.
Additionally, AtJAT3 and AtJAT4, localized at the plasma membrane and predominantly expressed in phloem companion cells, are responsible for JA import into target cells. These transporters play essential roles in systemic resistance, mediating the movement of JA between leaves during wound responses. In Arabidopsis, double mutants lacking JAT3 and JAT4 show impaired long-distance signaling and increased susceptibility to necrotrophic pathogens.
The importance of jasmonate transport becomes even more evident when considering its influence on metabolic flux. By directing where JA and its derivatives accumulate, transporters modulate whether the hormone is metabolized, stored, or used for signaling. For instance, peroxisomal exporter AtJAT2 and putative vacuolar transporters like AtABCG19 are likely to participate in downstream JA trafficking, although their specific roles are still under investigation.
In essence, jasmonate transporters act as gatekeepers, ensuring the spatial precision of hormone action. Without them, even a properly synthesized JA molecule may never reach its site of action, making these transport proteins indispensable to the hormone’s overall function.
Jasmonate Signaling: From Wounds to Gene Expression
The jasmonate signaling pathway exemplifies the sophisticated regulatory networks plants use to respond rapidly to internal and external cues. At the heart of this system lies jasmonoyl-isoleucine (JA-Ile), the bioactive form of jasmonic acid that orchestrates changes in gene expression in response to stress signals such as wounding, pathogen attack, and environmental fluctuations.
Once synthesized in the cytosol, JA-Ile must enter the nucleus to initiate transcriptional reprogramming. This process is facilitated by the AtJAT1 transporter, which imports JA-Ile into the nucleus, ensuring the hormone reaches its receptor complex. Inside the nucleus, JA-Ile binds to a co-receptor complex composed of the F-box protein COI1 (CORONATINE INSENSITIVE1) and the JAZ (JASMONATE-ZIM DOMAIN) transcriptional repressors.
Under normal conditions, JAZ proteins suppress the activity of key transcription factors such as MYC2, MYC3, and MYC4, which are responsible for activating a wide array of JA-responsive genes. When JA-Ile levels rise—often in response to stress—this hormone binds to the SCF^COI1-JAZ complex, triggering ubiquitination and subsequent degradation of JAZ proteins via the 26S proteasome. The degradation of JAZ releases MYC transcription factors, allowing them to engage with the transcriptional machinery, including RNA polymerase II and coactivators like MED25, to initiate gene expression.
Notably, this signaling cascade includes built-in negative feedback loops. Some JA-responsive genes encode new JAZ repressors or enzymes that degrade JA-Ile, ensuring the signal is temporary and tightly controlled. This dynamic balance between activation and repression allows plants to respond rapidly but also return to homeostasis once the threat is mitigated.
Overall, the JA-Ile signaling pathway is a model of signal perception, amplification, and regulation, enabling plants to translate external stress into a coordinated molecular response. Its precision hinges on proper nuclear transport and tightly regulated protein-protein interactions.
Looking Ahead: Why Jasmonate Transport Matters for Agriculture and Evolution
Understanding the transport of jasmonates offers a transformative perspective on both plant physiology and practical applications in agriculture. While much attention has been given to jasmonate biosynthesis and signaling, it is now clear that transport processes are equally essential, functioning as the gatekeepers that ensure jasmonates act in the right place at the right time. This insight opens new frontiers for enhancing plant resilience, crop productivity, and stress tolerance.
In agricultural systems increasingly threatened by climate change, the ability to manipulate jasmonate transporters—such as JAT1, JAT3, and JAT4—may allow researchers to fine-tune plant responses to wounding, drought, salinity, and pathogen attack. For instance, boosting the expression of transporters that support systemic JA movement could lead to crops with enhanced immunity and faster wound signaling, without the growth penalties associated with constant hormone activation. Conversely, modulating JA export or degradation could help reduce stress overreaction, conserving energy for growth and reproduction.
Beyond agriculture, jasmonate transport also offers an evolutionary lens into how plants have adapted to land environments. In early land plants like Marchantia polymorpha, signaling molecules such as dn-OPDA (dinor-OPDA) perform similar roles to JA-Ile in higher plants. This suggests that the evolution of JA-Ile-based signaling systems was accompanied by the emergence of sophisticated transport mechanisms, enabling more localized and systemic responses.
Ongoing research aims to characterize the substrate specificity, directionality, and regulatory control of each jasmonate transporter. Understanding these fine-tuned logistics could allow for the engineering of synthetic transport pathways or the reprogramming of hormonal flow in crops, pushing the boundaries of plant biotechnology. As we uncover more about how jasmonates move within and between cells, we inch closer to designing crops that are not only high-yielding but also exceptionally responsive and resilient.
References
Browse, J. (2009). Jasmonate passes muster: a receptor and targets for the defense hormone. Annual Review of Plant Biology, 60, 183–205.
https://doi.org/10.1146/annurev.arplant.043008.092007
Li, M., Yu, G., Cao, C., & Liu, P. (2021). Metabolism, signaling, and transport of jasmonates. Plant Communications, 2(9), 100231.
https://doi.org/10.1016/j.xplc.2021.100231
Wasternack, C., & Hause, B. (2013). Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Annals of Botany, 111(6), 1021–1058.
https://doi.org/10.1093/aob/mct067
Howe, G. A., Major, I. T., & Koo, A. J. (2018). Modularity in jasmonate signaling for multistress resilience. Annual Review of Plant Biology, 69, 387–415.
https://doi.org/10.1146/annurev-arplant-042817-040047
Wasternack, C., & Feussner, I. (2018). The oxylipin pathways: biochemistry and function. Annual Review of Plant Biology, 69, 363–386.
https://doi.org/10.1146/annurev-arplant-042817-040440
Koo, A. J. (2018). Metabolism of the plant hormone jasmonate: a sentinel for tissue damage and master regulator of stress response. Phytochemistry Reviews, 17, 51–80.
https://doi.org/10.1007/s11101-017-9510-8
Guan, L., Denkert, N., Eisa, A., et al. (2019). JASSY, a chloroplast outer membrane protein required for jasmonate biosynthesis. Proceedings of the National Academy of Sciences, 116(21), 10568–10575.
https://doi.org/10.1073/pnas.1901456116
Li, Q., Zheng, J., Li, S., et al. (2017). Transporter-mediated nuclear entry of jasmonoyl-isoleucine is essential for jasmonate signaling. Molecular Plant, 10(5), 695–708.
https://doi.org/10.1016/j.molp.2017.03.008
Li, M., Wang, F., Li, S., et al. (2020). Importers drive leaf-to-leaf jasmonic acid transmission in wound-induced systemic immunity. Molecular Plant, 13(10), 1485–1498.
https://doi.org/10.1016/j.molp.2020.07.008
Chini, A., Fonseca, S., Fernandez, G., et al. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature, 448(7154), 666–671.
https://doi.org/10.1038/nature06006
Sheard, L. B., Tan, X., Mao, H., et al. (2010). Jasmonate perception by inositol-phosphate-potentiated COI1–JAZ co-receptor. Nature, 468(7322), 400–405.
https://doi.org/10.1038/nature09430
Monte, I., Ishida, S., Zamarreño, Á. M., et al. (2018). Ligand-receptor co-evolution shaped the jasmonate pathway in land plants. Nature Chemical Biology, 14(5), 480–488.