Targeting BTK: The Science Behind a Game-Changer in Cancer and Autoimmune Therapy
Abstract
Bruton’s tyrosine kinase (BTK) has emerged as a central regulator of immune signaling, originally linked to X-linked agammaglobulinemia and now a major therapeutic target across oncology, autoimmunity, and infectious diseases. This blog explores BTK’s pivotal role in B-cell receptor signaling and its expanding influence in T cells, macrophages, and inflammatory cascades. We highlight the clinical success of BTK inhibitors like ibrutinib in B-cell malignancies, examine ongoing trials in autoimmune conditions such as systemic lupus erythematosus and multiple sclerosis, and discuss novel uses in hyperinflammatory states like COVID-19. As resistance to first-generation inhibitors grows, newer, more selective compounds are reshaping the landscape of targeted immunotherapy. The future of BTK-directed therapy promises improved precision, broader disease coverage, and reduced side effects—marking a new era in immune modulation.
Bruton’s Tyrosine Kinase: The Unsung Hero of Modern Immunology
In the intricate web of immune system signaling, few molecules have captured as much scientific and clinical interest in recent years as Bruton’s tyrosine kinase (BTK). This non-receptor TEC family kinase, once primarily known for its association with a rare immunodeficiency, is now recognized as a central player in immunoregulation and a prime therapeutic target in both hematological malignancies and autoimmune diseases.
The story of BTK began in 1952 when pediatrician Ogden Bruton first described a rare condition now known as X-linked agammaglobulinemia (XLA). Decades later, in 1993, BTK mutations were identified as the underlying cause of XLA, unveiling the enzyme’s essential role in B-cell development. Without functional BTK, patients fail to produce mature B cells and immunoglobulins, leading to recurrent infections and profound immunodeficiency.
Fast-forward to today, and BTK has become a therapeutic cornerstone. BTK inhibitors like ibrutinib and acalabrutinib have transformed the management of chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL), offering targeted, chemotherapy-free options. The field has expanded even further to explore BTK’s involvement in autoimmune disorders such as systemic lupus erythematosus (SLE) and multiple sclerosis (MS), as well as its potential to curb inflammation in severe COVID-19 cases.
What’s driving this surge in BTK-focused innovation? The answer lies in its versatile immune function. BTK is now understood to operate not only in B cells but also in macrophages, neutrophils, mast cells, and even T cells, linking it to both innate and adaptive immunity. This makes it a uniquely attractive target for diverse clinical applications, particularly where immune dysregulation is at play.
With newer, more selective BTK inhibitors emerging and novel clinical applications on the horizon, BTK is poised to remain at the forefront of immunological research and therapeutic development.
BTK’s Central Role in the Immune System: The Signal Conductor of B Cells and Beyond
At the heart of adaptive immunity lies a cascade of signaling events that determine the fate of B cells—whether they survive, mature, or undergo apoptosis. Among the pivotal regulators of these processes is Bruton’s tyrosine kinase (BTK). Far from being a one-trick enzyme, BTK orchestrates multiple signaling pathways across both adaptive and innate immune cells, making it indispensable for immune homeostasis and response.
The most well-characterized role of BTK is within the B-cell receptor (BCR) signaling pathway. Upon antigen recognition, BCR activation initiates a chain reaction: BTK is recruited to the plasma membrane via its Pleckstrin Homology (PH) domain, where it is phosphorylated—first at Y551 by kinases like SYK or LYN, and subsequently at Y223 via autophosphorylation. Once activated, BTK phosphorylates PLCγ2, leading to the generation of secondary messengers like DAG and IP3. This results in the activation of downstream pathways such as NF-κB, NFAT, and MAPK, ultimately driving B-cell proliferation, differentiation, and antibody production.
But BTK’s influence extends far beyond B cells. It plays a key role in Toll-like receptor (TLR) signaling, especially in macrophages and dendritic cells. By interacting with adaptor molecules like MYD88 and IRAK1, BTK helps regulate inflammatory responses, bridging the gap between innate and adaptive immunity.
BTK also participates in chemokine receptor signaling, such as through CXCR4, which controls immune cell trafficking and localization. Defects in BTK expression or function can disrupt these processes, contributing to immunodeficiency or autoimmunity.
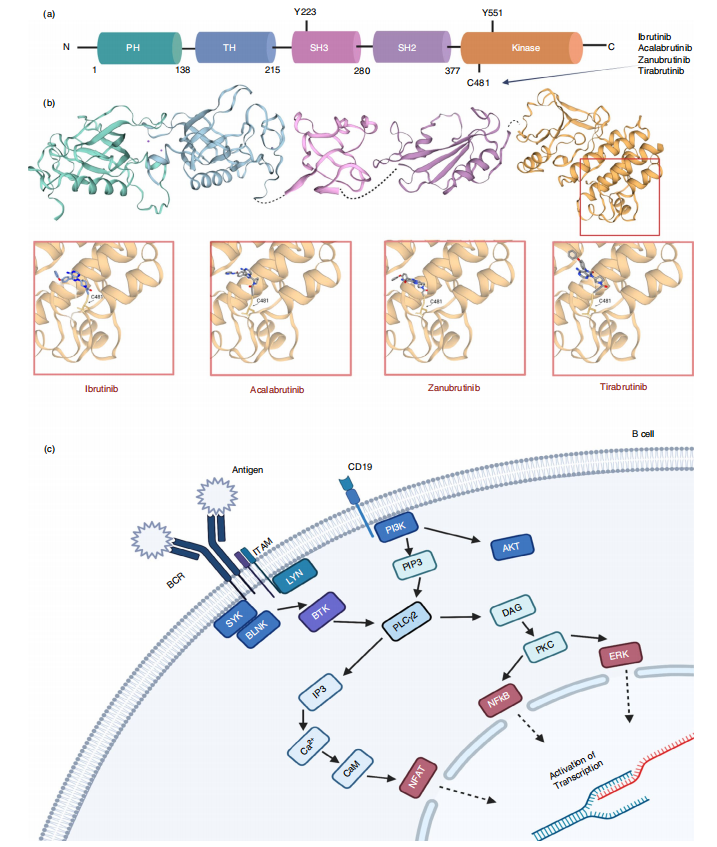
FIig. 1 Structural overview of BTK and BTK inhibitors and its position within the B-cell receptor signalling pathway
Given its centrality to immune activation and regulation, BTK is more than just a component of B-cell signaling—it is a critical immune integrator. This broad functional spectrum explains why BTK inhibitors have therapeutic implications across oncology, immunology, and infectious diseases.
BTK Beyond B Cells: The Silent Driver in T Cells, Macrophages, and Inflammatory Signaling
While Bruton’s tyrosine kinase (BTK) is most famously linked to B-cell signaling, growing evidence reveals its broader immunological reach. This kinase, once considered exclusive to adaptive immunity, now stands at the crossroads of both innate and adaptive immune responses, influencing cell behavior across a wide range of leukocyte populations.
T Cells: Modulating Immunotherapy Potential
BTK is not highly expressed in T cells, but its inhibition still impacts their function, especially in the context of cancer. Studies have shown that BTK inhibitors like ibrutinib indirectly enhance T-cell activity by altering the tumor microenvironment. In patients with chronic lymphocytic leukemia (CLL), ibrutinib therapy increases CD8⁺ T-cell numbers, enhances cytokine production, and boosts T-cell receptor diversity—factors that collectively support a more robust anti-tumor response. This makes BTK inhibition a potential complement to immune checkpoint inhibitors.
Macrophages and Dendritic Cells: Shaping Innate Immunity
BTK is abundantly expressed in myeloid cells such as macrophages and dendritic cells, where it mediates Fc receptor and Toll-like receptor (TLR) signaling. For example, ibrutinib has been shown to suppress cytokine production in monocytes without impairing phagocytosis, suggesting a way to curb inflammation while preserving antimicrobial defenses. Additionally, nurse-like cells (NLCs)—macrophage-like cells in the CLL microenvironment—depend on BTK for their immunosuppressive function. Inhibiting BTK in these cells reduces their support for leukemic survival.
Inflammation and the Inflammasome Axis
BTK also plays a pivotal role in the NLRP3 inflammasome, a critical component of inflammatory signaling. By promoting phosphorylation events and recruiting adaptor proteins like ASC, BTK triggers caspase-1 activation and IL-1β production. This positions BTK as a valuable target not just in cancer, but in chronic inflammatory conditions and autoimmunity as well.
Together, these insights reflect a paradigm shift: BTK is not just a B-cell kinase, but a multifunctional regulator with far-reaching implications in immunity and inflammation.
From Cancer to COVID-19: The Expanding Clinical Reach of BTK Inhibition
The therapeutic spotlight on Bruton’s tyrosine kinase (BTK) has grown significantly in recent years, not only due to its role in B-cell malignancies, but also because of its emerging applications in autoimmune diseases and even infectious disease management. This broad clinical relevance stems from BTK’s central role in immune cell signaling and inflammation.
Cancer: Targeting BTK in B-cell Malignancies
BTK is a well-established driver in several lymphoproliferative disorders, particularly chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), and Waldenström’s macroglobulinemia (WM). The approval of ibrutinib, a covalent BTK inhibitor, marked a turning point in treatment strategies for these cancers. Ibrutinib works by irreversibly binding to the C481 residue of BTK, thereby disrupting BCR signaling and inducing cancer cell death. However, drug resistance—often due to C481S mutations—and off-target effects like atrial fibrillation and bleeding have prompted the development of second-generation inhibitors (e.g., acalabrutinib, zanubrutinib) with improved specificity.
Autoimmune Diseases: Taming the Overactive Immune Response
Beyond oncology, BTK inhibition is showing promise in autoimmune conditions like systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and multiple sclerosis (MS). In these diseases, BTK contributes to aberrant B-cell activation, autoantibody production, and pro-inflammatory signaling. Inhibitors such as evobrutinib, branebrutinib, and tolebrutinib are in various stages of clinical trials, demonstrating immunomodulatory effects without broadly suppressing immunity.
COVID-19: A Surprising Application
Perhaps one of the most unexpected and urgent clinical frontiers for BTK inhibitors has been the COVID-19 pandemic. Severe COVID-19 is marked by excessive macrophage activation and cytokine storm, both of which are influenced by BTK. Trials using acalabrutinib in hospitalized patients showed improvements in oxygenation and reductions in inflammatory markers, suggesting that BTK inhibition may offer a novel anti-inflammatory strategy in viral infections.
From oncology to virology, BTK’s therapeutic impact is expanding—and reshaping how we think about immune modulation.
BTK Inhibition in the Clinic: From Cancer Therapies to COVID-19 Interventions
Bruton’s tyrosine kinase (BTK) has evolved from a niche interest in immunodeficiency to a high-value therapeutic target across multiple disease areas. Its role as a signaling hub in both adaptive and innate immunity underpins its clinical versatility, particularly in oncology, autoimmunity, and inflammatory diseases like COVID-19.
Cancer: Redefining Treatment in B-cell Malignancies
BTK inhibitors have transformed the treatment landscape for B-cell cancers such as chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), and Waldenström’s macroglobulinemia (WM). The first-generation inhibitor ibrutinib irreversibly binds to the C481 residue in the BTK kinase domain, disrupting B-cell receptor signaling and inducing apoptosis in malignant cells. It offered a breakthrough alternative to chemotherapy, with significant improvements in progression-free survival.
However, ibrutinib is not without limitations. Off-target toxicities, particularly bleeding and atrial fibrillation, along with acquired resistance—often driven by C481S mutations—have spurred the development of next-generation inhibitors like acalabrutinib and zanubrutinib, which provide greater selectivity and better tolerability.
Autoimmune Disorders and Beyond
BTK’s role in modulating immune responses has also made it a candidate for treating autoimmune diseases. In systemic lupus erythematosus (SLE), BTK promotes the activation of autoreactive B cells and pro-inflammatory cytokine production. Inhibition of BTK in preclinical models has shown promising reductions in disease activity. Clinical trials for agents like evobrutinib and tolebrutinib are underway in diseases such as SLE, rheumatoid arthritis, and multiple sclerosis, aiming to reduce immune overactivity without full immunosuppression.
COVID-19: A New Frontier
During the COVID-19 pandemic, BTK inhibitors unexpectedly found relevance. In patients with severe disease, hyperinflammatory macrophage activation contributes to lung damage. Early studies using acalabrutinib reported improved oxygenation and reduced inflammation in hospitalized patients, suggesting that BTK blockade could serve as a novel anti-inflammatory approach in viral-induced hyperinflammation.
From hematology to virology, BTK continues to prove its broad therapeutic potential.
Future Horizons: Next-Generation BTK Inhibitors and New Therapeutic Frontiers
As the landscape of immunotherapy evolves, so too does the science of BTK inhibition. While first-generation inhibitors like ibrutinib revolutionized treatment for B-cell malignancies, challenges such as drug resistance, off-target effects, and limited applications in solid tumors have spurred a new wave of innovation. Today, researchers are focused on developing next-generation BTK inhibitors that promise greater specificity, enhanced safety, and broader clinical utility.
Overcoming Resistance with Reversible Inhibitors
One of the key limitations of ibrutinib is the emergence of C481S mutations in the BTK binding site, which prevent irreversible inhibitors from functioning effectively. In response, non-covalent, reversible inhibitors like pirtobrutinib (LOXO-305) have been developed. Unlike their covalent counterparts, these agents do not rely on the C481 residue and can maintain efficacy even in resistant patient populations. Pirtobrutinib has shown promising results in early-phase trials, including activity against ibrutinib- and venetoclax-resistant chronic lymphocytic leukemia (CLL), without the significant side effects associated with first-generation compounds.
Expanding Indications Beyond Hematology
Beyond cancer, BTK inhibitors are increasingly being tested in autoimmune diseases and neuroinflammatory conditions. One promising compound, tolebrutinib, is a brain-penetrant BTK inhibitor currently under investigation for multiple sclerosis (MS). Its ability to access the central nervous system could offer new hope for patients with progressive MS, where inflammation and microglial activation play key roles.
Similarly, remibrutinib, designed for high specificity and minimal off-target effects, is being evaluated for urticaria and Sjögren’s syndrome, reflecting a broader push to use BTK modulation to treat chronic inflammatory diseases.
Toward Personalized BTK Therapy
Looking ahead, personalized medicine strategies—such as mutation profiling and combination therapies—may further enhance the clinical success of BTK inhibitors. Whether through dual-targeting approaches or precision biomarker selection, the next chapter in BTK research is poised to unlock more tailored, durable, and safer therapies.
References
McDonald, C., Xanthopoulos, C., & Kostareli, E. (2021). The role of Bruton’s tyrosine kinase in the immune system and disease. Immunology, 164(4), 722–736.
https://doi.org/10.1111/imm.13416
Burger, J. A., & Wiestner, A. (2018). Targeting B cell receptor signalling in cancer: Preclinical and clinical advances. Nature Reviews Cancer, 18(3), 148–167.
https://doi.org/10.1038/nrc.2017.121
Rawlings, D. J., Schwartz, M. A., Jackson, S. W., & Meyer-Bahlburg, A. (2012). Integration of B cell responses through Toll-like receptors and antigen receptors. Nature Reviews Immunology, 12(4), 282–294.
https://doi.org/10.1038/nri3150
Mato, A. R., Shah, N. N., Jurczak, W., et al. (2021). Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. The Lancet, 397(10277), 892–901.
https://doi.org/10.1016/S0140-6736(21)00224-5
Roschewski, M., Lionakis, M. S., Sharman, J. P., et al. (2020). Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Science Immunology, 5(48), eabd0110.
https://doi.org/10.1126/sciimmunol.abd0110
Dörner, T., & Furie, R. (2019). Novel paradigms in systemic lupus erythematosus. The Lancet, 393(10188), 2344–2358.
https://doi.org/10.1016/S0140-6736(19)30546-X
Dolgin, E. (2021). BTK blockers make headway in multiple sclerosis. Nature Biotechnology, 39(1), 3–5.