Selatinib Explained: A Next-Gen Tyrosine Kinase Inhibitor Targeting HER2 and EGFR
Abstract
Selatinib is an emerging dual tyrosine kinase inhibitor (TKI) targeting EGFR and HER2, two critical oncogenic drivers in cancers such as HER2-positive breast and gastric cancers. As a structurally optimized analog of lapatinib, Selatinib offers improved pharmacokinetics, reduced toxicity, and promising antitumor activity. Early clinical trials have demonstrated its safety and bioavailability, while preclinical data highlight its effectiveness across multiple cancer types, including potential in RET-mutated lung adenocarcinoma. With ongoing research and in silico validation, Selatinib holds promise as a next-generation therapeutic agent in precision oncology. This article explores its mechanism of action, clinical development, cancer applications, and future outlook in targeted cancer therapy.
Introduction: What Is Selatinib?
Selatinib is an emerging small-molecule drug currently under investigation for its potential in targeted cancer therapy. It functions as a dual tyrosine kinase inhibitor (TKI) that specifically targets the epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) — two proteins often overexpressed or mutated in various cancers, especially HER2-positive breast cancer. As resistance to first-generation TKIs becomes increasingly common, novel inhibitors like Selatinib represent the next generation of more selective and effective treatments.
What sets Selatinib apart is its structural derivation from Lapatinib, a well-known dual EGFR/HER2 inhibitor. Researchers have chemically modified Selatinib to enhance its pharmacokinetic properties, including better bioavailability, metabolic stability, and target affinity. This makes Selatinib a strong candidate for treating cancers where HER2 plays a critical oncogenic role.
Preclinical studies and early clinical trials suggest that Selatinib may be better tolerated and more effective than its predecessors. A Phase I clinical trial (NCT01931943) evaluated its safety, tolerability, and pharmacokinetics in healthy volunteers, laying the groundwork for further trials in cancer patients. Additionally, molecular modeling and binding affinity studies indicate that Selatinib interacts favorably with key residues in EGFR and HER2, suggesting a promising therapeutic mechanism.
With the rise of precision oncology, drugs like Selatinib that target specific molecular drivers are becoming increasingly important. Although it has not yet received FDA approval, Selatinib remains under active investigation as a potential breakthrough in HER2-driven cancer treatment.
How Selatinib Works: Mechanism of Action
Selatinib functions as a dual tyrosine kinase inhibitor (TKI) that specifically targets two key receptors involved in cancer progression: epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2). These receptors are members of the ErbB family of receptor tyrosine kinases, which regulate crucial cellular processes such as proliferation, survival, angiogenesis, and migration. When overexpressed or mutated — particularly in breast, gastric, and lung cancers — these receptors can drive tumorigenesis and metastasis.
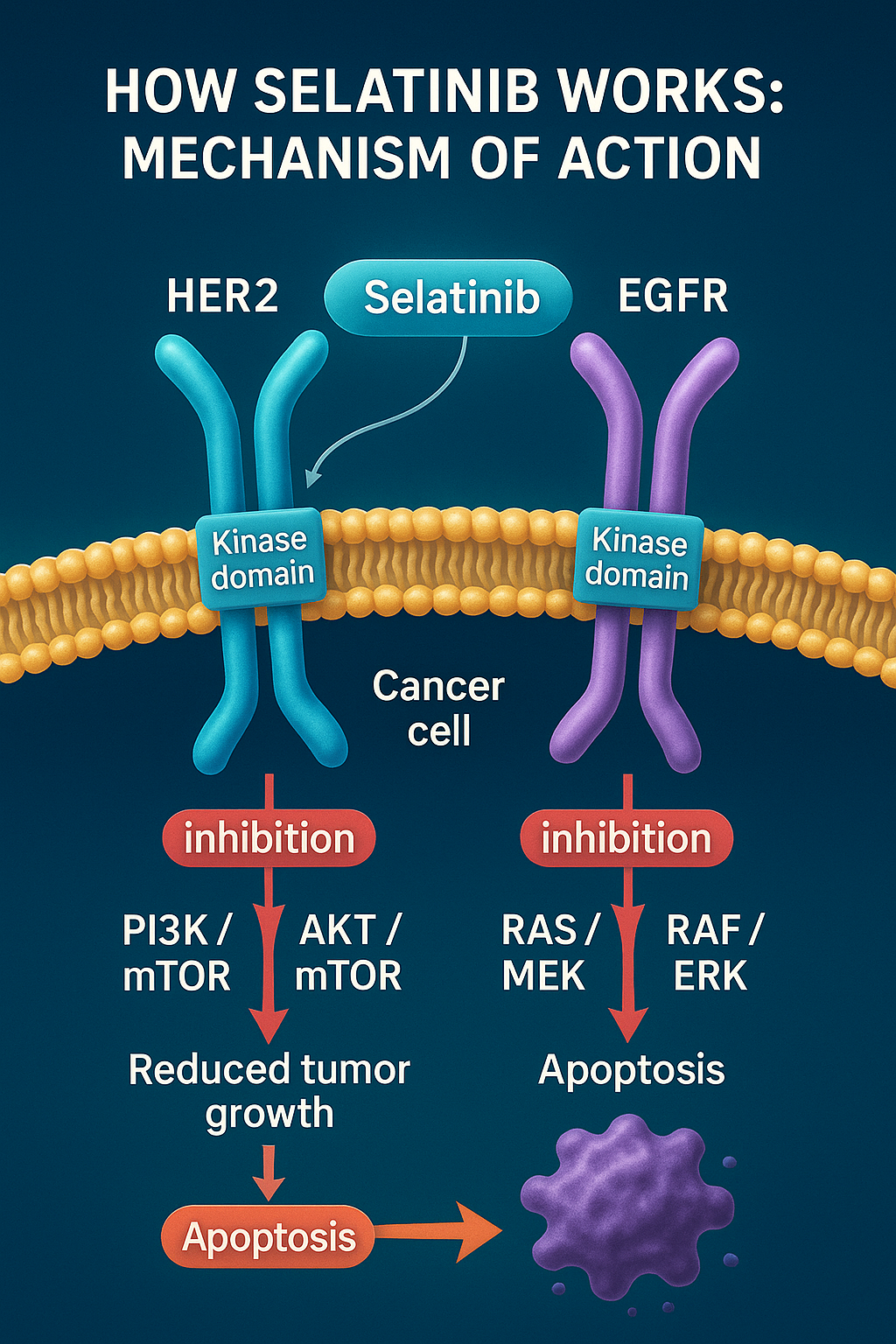
Selatinib exerts its therapeutic effect by blocking the intracellular kinase domain of EGFR and HER2. This inhibition prevents autophosphorylation and subsequent activation of downstream signaling pathways, such as PI3K/AKT/mTOR and RAS/RAF/MEK/ERK, both of which are central to cancer cell survival and proliferation. By suppressing these pathways, Selatinib reduces tumor growth, induces apoptosis, and may overcome resistance to earlier-generation TKIs like lapatinib.
What distinguishes Selatinib is its higher binding affinity and selectivity toward mutated and overactive HER2/EGFR, as demonstrated in structural docking simulations and preclinical enzymatic assays. Furthermore, it has shown improved pharmacodynamic stability and reduced off-target toxicity in early studies, making it a promising candidate for tumors that are unresponsive to other HER2-targeted agents.
Selatinib also appears to interfere with HER2 heterodimerization with other receptors in the ErbB family, a mechanism known to contribute to drug resistance. This makes Selatinib particularly attractive for combination therapy strategies or as a second-line option in patients who have failed HER2 monoclonal antibody therapies such as trastuzumab.
Clinical Trials and Research Status
Selatinib has undergone early-phase clinical evaluation to determine its safety, pharmacokinetics, and preliminary efficacy as a targeted cancer therapy. The most notable trial to date is a Phase I, single-ascending-dose study (NCT01931943), which aimed to evaluate Selatinib in healthy adult volunteers. This trial, published in Investigational New Drugs, confirmed that Selatinib exhibits a favorable safety profile, with no dose-limiting toxicities observed across multiple escalating doses.
The study also highlighted Selatinib’s oral bioavailability and its dose-proportional pharmacokinetics, with rapid absorption and a plasma half-life suitable for once-daily dosing. Importantly, the drug was metabolized into lapatinib, suggesting that Selatinib may serve as both a standalone TKI and a prodrug with therapeutic synergy.
Beyond this initial trial, Selatinib has been featured in preclinical tumor models, where it demonstrated strong antiproliferative activity in HER2-overexpressing cell lines. It has shown promising results in breast cancer, gastric cancer, and potentially in lung adenocarcinoma harboring RET mutations — expanding its utility beyond traditional HER2-positive contexts.
Recent computational and molecular docking studies further support its binding efficiency and selectivity toward the kinase domains of EGFR and HER2, reinforcing its mechanism of action and validating its drug-target interactions at the molecular level.
As of now, no Phase II or III trials have been publicly registered. However, ongoing molecular simulation, combination therapy modeling, and resistance pathway research continue to strengthen the rationale for its further clinical advancement.
Potential Uses and Cancer Types Targeted
Selatinib is being explored as a promising agent in the treatment of cancers driven by overexpression or mutation of HER2 and EGFR, two key oncogenic drivers. Its dual-inhibition mechanism positions it as a viable treatment for multiple HER2-positive malignancies, especially in cases where existing therapies have failed or resistance has developed.
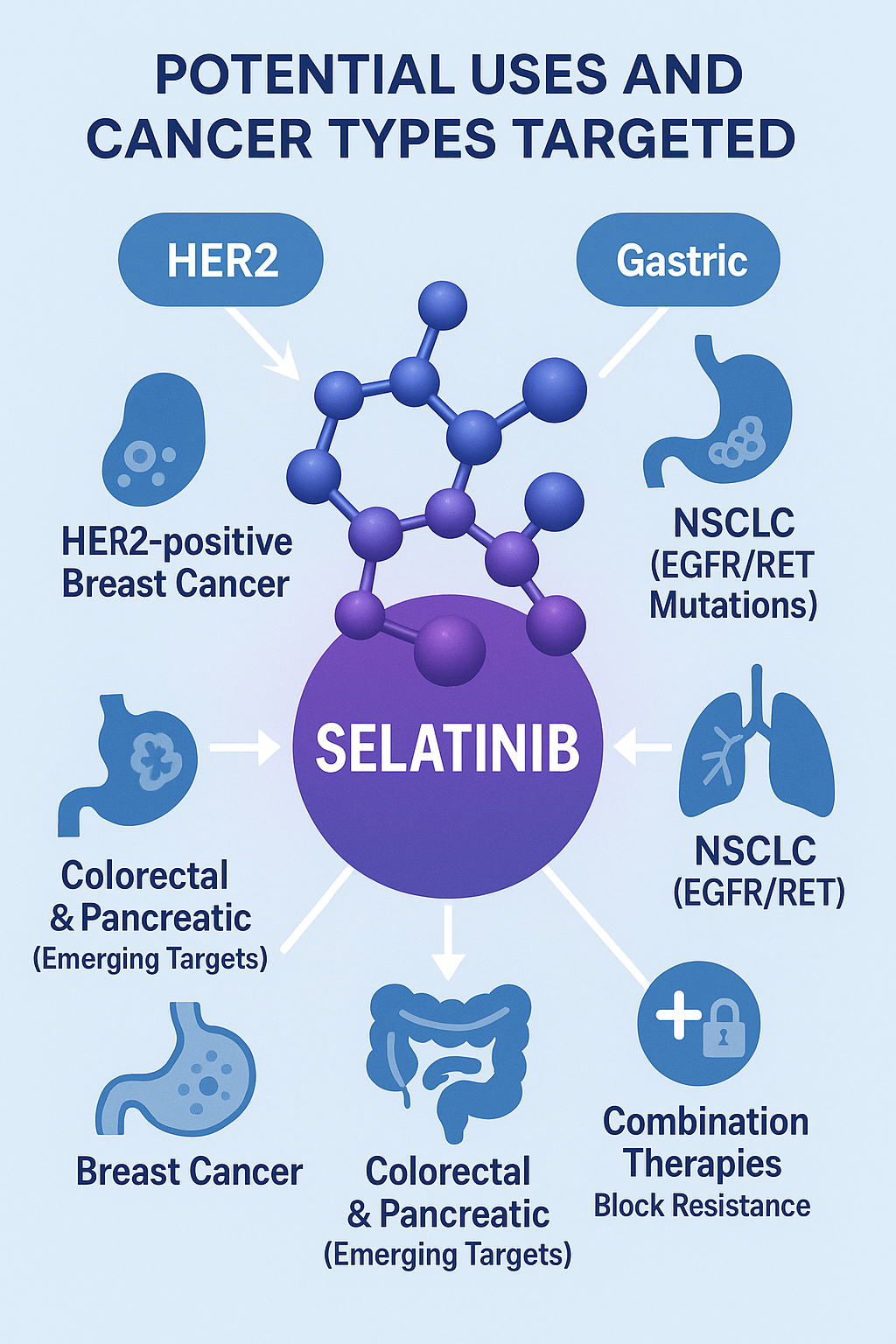
The primary therapeutic focus of Selatinib has been HER2-positive breast cancer, a subtype accounting for approximately 15–20% of all breast cancer cases. Preclinical studies have demonstrated that Selatinib significantly inhibits the proliferation of HER2-overexpressing breast cancer cells, with improved efficacy compared to earlier TKIs such as lapatinib. This makes it a potential option for second-line therapy or in combination with monoclonal antibodies like trastuzumab.
Beyond breast cancer, Selatinib has shown preclinical activity in HER2-amplified gastric cancer, where it disrupts downstream oncogenic signaling and reduces tumor viability. Its potent EGFR inhibition also suggests application in non-small cell lung cancer (NSCLC), especially among subtypes harboring RET or EGFR mutations. One study even identified Selatinib’s capacity to interact with RET-mutated kinases, expanding its utility in RET-driven lung adenocarcinomas.
In molecular docking and in silico modeling, Selatinib displayed strong interactions with kinase targets involved in colorectal and pancreatic cancers, although clinical validation in these areas remains pending. Its favorable pharmacokinetics, coupled with its ability to bypass resistance pathways like HER2 dimerization, make it an attractive candidate for combination therapies involving chemotherapy, immune checkpoint inhibitors, or PI3K inhibitors.
In summary, Selatinib’s flexibility across multiple cancer types underscores its value as a multi-targeted oncology drug that may redefine treatment in HER2-driven and kinase-mutated malignancies.
Conclusion: Future of Selatinib in Oncology
Selatinib stands at the forefront of a new wave of targeted cancer therapies, offering a refined, dual-inhibition approach against two of the most prominent oncogenic drivers: EGFR and HER2. With encouraging preclinical results and successful Phase I human trials demonstrating tolerability, favorable pharmacokinetics, and bioactivity, Selatinib is poised to address key gaps in current treatment regimens for HER2-positive and kinase-driven tumors.
Its ability to overcome some of the limitations of first-generation TKIs—such as poor solubility, off-target toxicity, and resistance mechanisms like HER2 heterodimerization—positions Selatinib as a viable next-generation candidate. In particular, it offers promise in HER2-positive breast and gastric cancers, and emerging research suggests potential roles in RET-mutated lung adenocarcinoma and other kinase-reliant malignancies.
However, Selatinib is still in early-stage development. For it to gain regulatory approval and clinical adoption, it must progress through Phase II/III clinical trials that establish efficacy in real-world cancer populations. Future directions include evaluating Selatinib in combination therapies, especially alongside monoclonal antibodies (e.g., trastuzumab), immunotherapies, or PI3K/mTOR inhibitors.
Furthermore, computational studies support its utility in drug repurposing strategies and personalized medicine approaches, where kinase mutation profiles guide treatment selection. As oncology shifts toward precision targeting, drugs like Selatinib—engineered with specificity, reduced toxicity, and broad applicability—represent a paradigm shift in cancer care.
If successful, Selatinib could become a cornerstone molecule in the targeted therapy arsenal, particularly for patients with limited options after developing resistance to current HER2 or EGFR-directed agents.
References
Wang MN, Kuang Y, Gong LY, Hua Y, Pei Q, Guo CX, Cao Y, Huang J, Yang GP. First-in-human, phase I single-ascending-dose study of the safety, pharmacokinetics, and relative bioavailability of selatinib, a dual EGFR-ErbB2 inhibitor in healthy subjects. Invest New Drugs. 2020 Dec;38(6):1826-1835. doi: 10.1007/s10637-020-00959-6. Epub 2020 Jun 13. PMID: 32535812; PMCID: PMC7575490.
https://link.springer.com/article/10.1007/s10637-020-00959-6
Lu M, Baima YJ, Ni Z, Yang L, Zhang SS, Zhang YT. Advances in the potential of nebulized inhalation for the treatment of pulmonary arterial hypertension. Curr Probl Cardiol. 2024 Oct;49(10):102752. doi: 10.1016/j.cpcardiol.2024.102752. Epub 2024 Jul 24. PMID: 39059783.
https://www.sciencedirect.com/science/article/abs/pii/S014628062400389X
Zhang L, Fan C, Guo Z, Li Y, Zhao S, Yang S, Yang Y, Zhu J, Lin D. Discovery of a potent dual EGFR/HER-2 inhibitor L-2 (selatinib) for the treatment of cancer. Eur J Med Chem. 2013 Nov;69:833-41. doi: 10.1016/j.ejmech.2013.09.032. Epub 2013 Sep 27. PMID: 24121234.
https://www.sciencedirect.com/science/article/abs/pii/S0223523413006053
Jiang JF, Chen XY, Zhong DF. [Metabolic research of domestically developed small molecule tyrosine kinase inhibitors]. Yao Xue Xue Bao. 2016 Feb;51(2):248-56. Chinese. PMID: 29856578.




