Rufinamide Explained: Uses, Mechanism, Side Effects & Clinical Benefits for Lennox-Gastaut Syndrome
Abstract
Rufinamide is an antiepileptic drug primarily used as adjunctive therapy for managing seizures associated with Lennox-Gastaut Syndrome (LGS), a severe form of childhood-onset epilepsy. It works by modulating voltage-gated sodium channels, stabilizing abnormal electrical activity in the brain. Rufinamide has demonstrated significant efficacy in reducing seizure frequency, particularly drop attacks, and is generally well tolerated with a relatively mild side effect profile. Approved for use in children and adults, it offers an important treatment option for individuals with refractory epilepsy. This blog post provides an overview of Rufinamide’s mechanism of action, clinical applications, safety considerations, and commonly asked questions to help patients, caregivers, and healthcare professionals better understand its role in epilepsy management.
What Is Rufinamide?
Rufinamide is an antiepileptic drug (AED) primarily used to manage seizures associated with Lennox-Gastaut Syndrome (LGS), a rare but severe form of epilepsy that typically begins in early childhood. First approved by the U.S. Food and Drug Administration (FDA) in 2008, Rufinamide represents an important option for patients who do not respond well to standard seizure medications.
Chemically classified as a triazole derivative, Rufinamide functions by modulating sodium channels in the brain, which helps stabilize abnormal electrical activity. Although its exact mechanism of action is still under study, Rufinamide is believed to prevent the excessive firing of neurons—an underlying cause of seizures. Unlike many other AEDs, it does not rely heavily on the liver’s cytochrome P450 enzymes, making it a more suitable choice for patients requiring combination therapy.
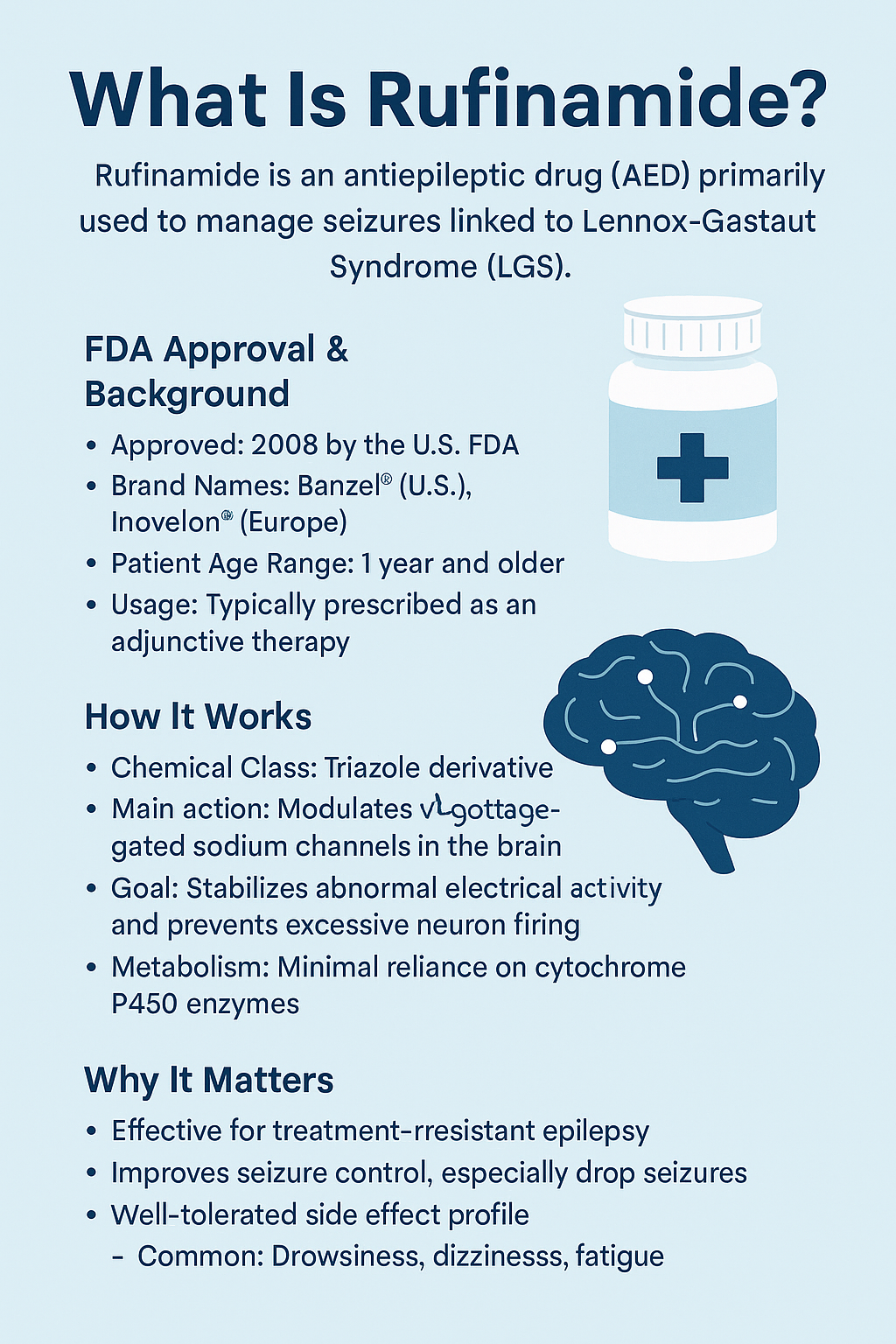
Rufinamide is most commonly marketed under the brand names Banzel® (in the U.S.) and Inovelon® (in Europe). It is typically prescribed as an adjunctive treatment, meaning it’s added to a regimen alongside other seizure medications to help improve control. It is approved for patients aged 1 year and older.
The importance of Rufinamide lies not only in its efficacy but also in its relatively tolerable side effect profile. Common side effects may include drowsiness, dizziness, and fatigue. For families and caregivers of individuals with LGS, Rufinamide provides hope for better seizure control and an improved quality of life.
In summary, Rufinamide is a valuable therapeutic agent in the complex management of Lennox-Gastaut Syndrome, helping reduce the frequency and severity of debilitating seizures.
How Rufinamide Works: Mechanism of Action
Understanding how Rufinamide works is essential for both healthcare professionals and caregivers managing epilepsy, especially Lennox-Gastaut Syndrome (LGS). Although its precise molecular mechanism isn’t fully understood, Rufinamide is believed to exert its antiepileptic effects primarily through modulation of voltage-gated sodium channels in the brain.
Neurons communicate through electrical impulses, and these impulses rely on the flow of sodium ions through specialized channels. In epileptic patients, this process becomes dysregulated, leading to excessive neuronal firing and seizures. Rufinamide is thought to prolong the inactive state of sodium channels, preventing them from reopening too soon and thereby reducing neuronal hyperexcitability.
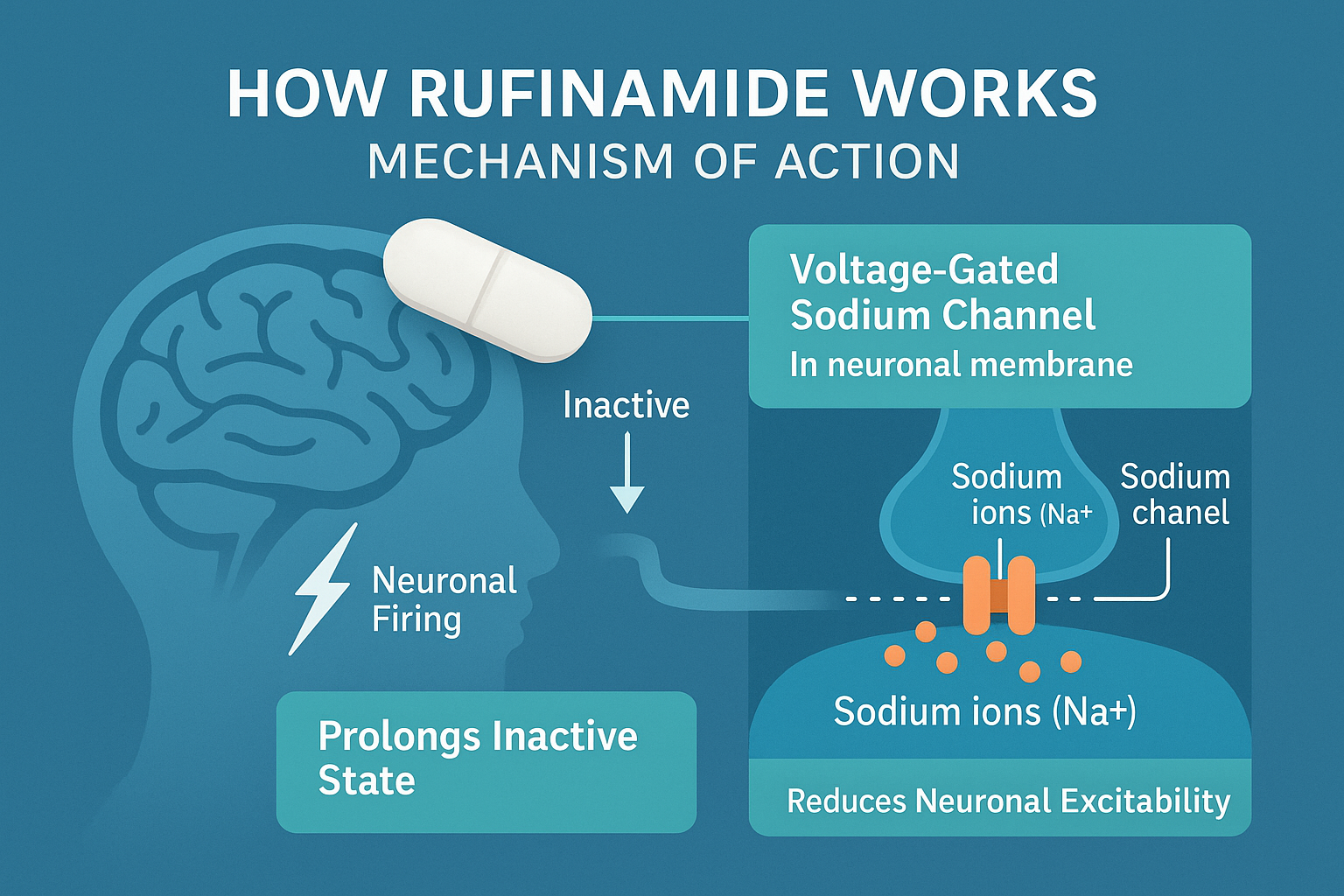
What sets Rufinamide apart from other antiepileptic drugs is its selectivity. It doesn’t broadly suppress brain activity, which helps preserve normal cognitive function while still controlling seizures. Additionally, Rufinamide has minimal interaction with cytochrome P450 enzymes, making it less likely to interfere with the metabolism of other medications—a key advantage for patients on multiple AEDs.
Recent studies suggest Rufinamide may also influence GABAergic transmission and other ion channels, although these pathways are still under investigation. Overall, its multi-faceted approach makes it a valuable adjunctive therapy, especially in hard-to-treat seizure syndromes.
In summary, Rufinamide’s unique mechanism—primarily targeting sodium channels—makes it effective in reducing seizure frequency without severely impacting overall brain function. This pharmacological profile supports its use in complex, treatment-resistant epilepsy cases.
Rufinamide in Clinical Use: Indications and Efficacy
Rufinamide is primarily approved as an adjunctive therapy for treating seizures associated with Lennox-Gastaut Syndrome (LGS) in individuals aged 1 year and older. LGS is one of the most severe forms of childhood-onset epilepsy, characterized by multiple types of seizures, including tonic, atonic, and atypical absence seizures. These are often resistant to treatment, making Rufinamide a valuable tool in the neurologist’s arsenal.
Rufinamide’s role is typically as a second-line or add-on therapy—it is rarely used as a monotherapy. It is often prescribed in combination with other antiepileptic drugs such as valproate, lamotrigine, or clobazam to enhance seizure control. Its broad-spectrum efficacy has been supported by multiple randomized controlled trials, showing significant reductions in seizure frequency, especially for drop attacks, which are common and dangerous in LGS patients.
Clinical studies have demonstrated that Rufinamide can reduce the frequency of drop seizures by over 40% compared to placebo, and in some patients, the improvement is even more substantial. The drug has also shown modest benefits in partial-onset seizures and other epilepsy types in off-label settings, though its FDA approval remains focused on LGS.
Furthermore, Rufinamide’s relatively mild side effect profile and low interaction potential with other medications make it well-tolerated for long-term use in both children and adults.
In conclusion, Rufinamide has emerged as a clinically effective and well-tolerated adjunctive treatment for one of the most challenging forms of epilepsy, significantly improving seizure management and patient outcomes.
Rufinamide Side Effects and Safety Profile
Like all antiepileptic medications, Rufinamide carries a range of potential side effects and safety considerations that users and caregivers should be aware of. Fortunately, Rufinamide is generally well tolerated, particularly when introduced gradually and taken with food to enhance absorption.
Common Side Effects
The most frequently reported side effects include:
Drowsiness or fatigue
Dizziness
Nausea or vomiting
Loss of coordination (ataxia)
Headache
These effects are typically dose-related and may diminish over time as the body adjusts to the medication. In pediatric populations, somnolence and behavioral changes such as irritability or agitation may also occur.
Serious and Rare Adverse Effects
While rare, more serious side effects have been observed:
Multiorgan hypersensitivity reactions
Stevens-Johnson Syndrome (SJS)
Shortening of the QT interval on ECG — unique to Rufinamide among AEDs
Suicidal ideation or behavior, a class warning for all antiepileptics
Due to QT shortening, patients with familial short QT syndrome should not take Rufinamide, and doctors often monitor ECGs, especially during dose titration.
Drug Interactions
Rufinamide has minimal impact on cytochrome P450 enzymes, meaning it poses fewer interactions than many older AEDs. However, enzyme-inducing AEDs like carbamazepine or phenytoin can reduce Rufinamide levels, and valproate can increase them—necessitating dosage adjustments.
In conclusion, Rufinamide is considered safe and tolerable for most individuals, but careful monitoring is essential, especially during the initial treatment phase. As always, patients should report any unusual symptoms to their healthcare provider.
Conclusion: Is Rufinamide Right for You?
Rufinamide is a valuable treatment option for managing Lennox-Gastaut Syndrome (LGS)—a complex and challenging form of epilepsy. As an adjunctive antiepileptic medication, it works by modulating sodium channels in the brain, helping to reduce excessive neuronal firing that leads to seizures. Its unique mechanism of action, relatively mild side effect profile, and limited drug interaction potential make it a suitable choice for many patients, particularly children with LGS.
While Rufinamide is not a cure for epilepsy, it has significantly improved seizure control and quality of life for many individuals. However, like all medications, it comes with risks and should be prescribed and monitored by a qualified healthcare provider. Ongoing communication between patients, families, and physicians is essential to ensure safe and effective treatment.
Whether you’re a caregiver, a healthcare professional, or someone living with epilepsy, understanding Rufinamide can help you make informed decisions. With continued research and clinical experience, this drug remains a critical component in the broader effort to manage treatment-resistant epilepsy.
If you believe Rufinamide might be a good fit, consult your neurologist to discuss its benefits, risks, and role in your treatment plan.
References
Glauser, T. A., Kluger, G., Sachdeo, R. C., Krauss, G. L., Perdomo, C., & Arroyo, S. (2008). Rufinamide for generalized seizures associated with Lennox–Gastaut syndrome. Neurology, 70(21), 1950–1958.
https://www.neurology.org/doi/abs/10.1212/01.wnl.0000303813.95800.0d
Rogawski, M. A., & Löscher, W. (2004). The neurobiology of antiepileptic drugs. Nature Reviews Neuroscience, 5(7), 553–564.
https://www.nature.com/articles/nrn1430
Perucca, E., & Tomson, T. (2011). The pharmacological treatment of epilepsy in adults. The Lancet Neurology, 10(5), 446–456.
https://www.thelancet.com/journals/laneur/article/PIIS1474-4422(11)70047-3/abstract
Johannessen Landmark, C. (2008). Antiepileptic drugs in non-epilepsy disorders: Relations between mechanisms of action and clinical efficacy. CNS Drugs, 22(1), 27–47.
https://link.springer.com/article/10.2165/00023210-200822010-00003