Ropivacaine: Pharmacology, Clinical Uses, and Related Compounds
Abstract
Ropivacaine is a long-acting amide local anesthetic widely used in various surgical procedures and pain management due to its favorable safety profile and effectiveness. This blog post provides an in-depth review of Ropivacaine, covering its pharmacology, clinical applications, and recent advancements. Ropivacaine operates by reversibly inhibiting sodium ion influx, resulting in selective sensory blockade with reduced motor impairment. It is preferred over Bupivacaine for its lower cardiotoxicity and CNS toxicity. Clinical studies demonstrate its efficacy in epidural, intrathecal, and peripheral nerve blocks, making it a versatile choice in anesthesia. Additionally, the synthesis and impurities associated with Ropivacaine, along with isotope-labeled compounds, are discussed, emphasizing their significance in pharmacokinetic studies and drug development. This comprehensive review aims to provide valuable insights into the multifaceted applications of Ropivacaine and its related chemical compounds.
Keywords: Ropivacaine, Local anesthetic, Pharmacology, Clinical applications, Chemical compounds
Introduction to Ropivacaine
Overview
Ropivacaine is a long-acting amide local anesthetic that has gained prominence in medical practice due to its efficacy and safety profile. It was developed as a pure S(-)-enantiomer to provide a safer alternative to racemic mixtures like Bupivacaine. Unlike Bupivacaine, Ropivacaine is less lipophilic, which translates to a lower risk of penetrating large myelinated motor fibers and a reduced likelihood of motor block and cardiotoxicity. This characteristic makes Ropivacaine a preferable choice for procedures where motor block is undesirable.
Mechanism of Action
Ropivacaine exerts its anesthetic effects by reversibly inhibiting the influx of sodium ions through sodium channels in neuronal membranes. This blockade prevents the propagation of nerve impulses, thereby inducing local anesthesia. The drug exhibits a degree of selectivity for sensory fibers over motor fibers due to its lower lipophilicity, which enhances its clinical utility by providing effective sensory block with minimal motor impairment. Additionally, Ropivacaine shows dose-dependent inhibition of potassium channels, further contributing to its anesthetic properties.
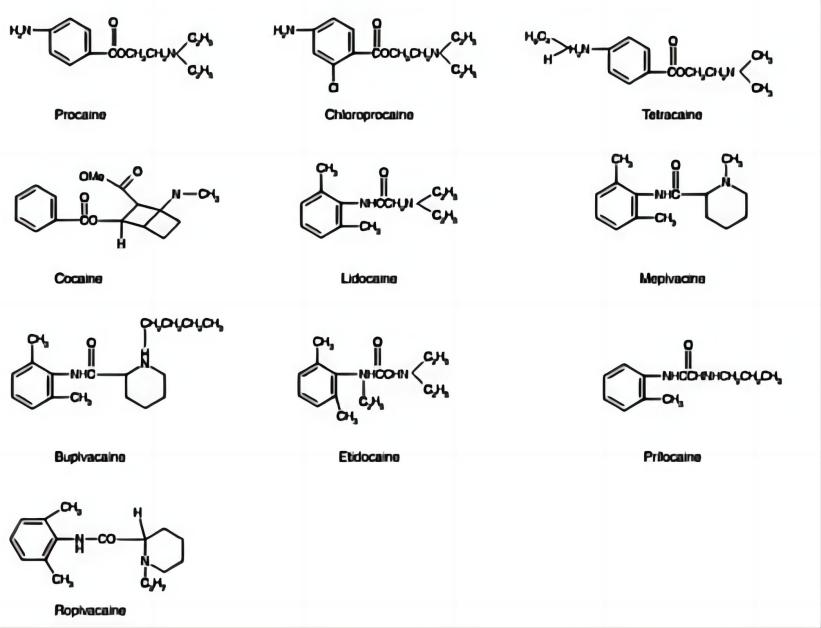
In comparison to Bupivacaine, Ropivacaine is associated with a significantly higher threshold for central nervous system (CNS) and cardiovascular toxicity. This is primarily due to its stereoselective properties and lower lipid solubility, which reduces its ability to cause adverse effects at clinically relevant doses.
Clinical Uses
Ropivacaine is widely used in various types of surgeries, including epidural anesthesia for abdominal, gynecological, and orthopedic surgeries. Its application in labor pain management is particularly noteworthy, where it provides effective analgesia with minimal motor block, allowing for better mobility during labor. Ropivacaine is also utilized in the management of chronic pain conditions through techniques such as nerve blocks and continuous infusion. Its favorable pharmacokinetic and pharmacodynamic profiles make it a versatile and valuable anesthetic in both acute and chronic pain management scenarios.
Pharmacology of Ropivacaine
Pharmacodynamics
Ropivacaine is known for its specific pharmacodynamic properties that make it a preferred local anesthetic in clinical practice. The primary action of Ropivacaine is the reversible inhibition of sodium ion influx through voltage-gated sodium channels in neuronal membranes. By blocking these channels, Ropivacaine prevents the initiation and conduction of nerve impulses, leading to a loss of sensation in the targeted area.
One of the notable features of Ropivacaine is its selective action on sensory nerves compared to motor nerves. This selectivity is largely due to its lower lipophilicity, which reduces its penetration into large myelinated motor fibers. Consequently, Ropivacaine provides effective pain relief with minimal motor block, which is particularly advantageous in procedures where motor function preservation is desired.
Ropivacaine’s impact on the central nervous system (CNS) and cardiovascular system is also significant. Studies have shown that Ropivacaine has a higher threshold for CNS toxicity compared to Bupivacaine, making it a safer option for patients. Similarly, its lower lipid solubility results in reduced cardiotoxicity, which is a critical factor in its clinical use. The stereoselective properties of Ropivacaine contribute to its reduced adverse effects, as the S(-)-enantiomer is less likely to cause CNS and cardiovascular complications.
Pharmacokinetics
The pharmacokinetics of Ropivacaine involve its absorption, distribution, metabolism, and excretion, which collectively determine its duration and intensity of action. Upon administration, Ropivacaine is absorbed into the bloodstream, with its plasma concentration influenced by the route of administration, dose, and the vascularity of the administration site.
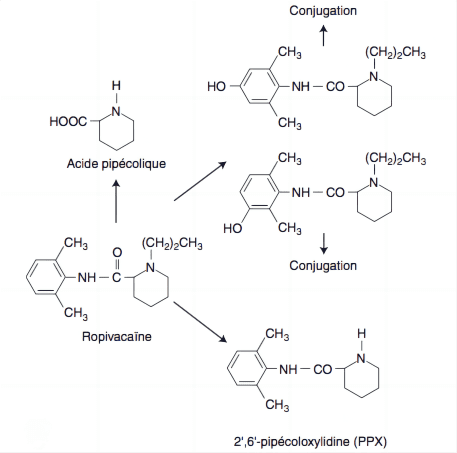
Ropivacaine exhibits dose-proportional pharmacokinetics up to an intravenous dose of 80 mg. Its distribution in the body is extensive, with approximately 94% of the drug bound to plasma proteins, primarily alpha-1-acid glycoprotein. This high protein binding capacity helps maintain a steady concentration of the drug in the plasma, prolonging its anesthetic effect.
Metabolism of Ropivacaine occurs predominantly in the liver, where it undergoes aromatic hydroxylation and N-dealkylation, primarily by cytochrome P450 enzymes CYP1A2 and CYP3A4. The primary metabolites, 3′-hydroxy-ropivacaine and 2′,6′-pipecoloxylidide, are less active and are further processed for excretion.
Excretion of Ropivacaine is mainly through the kidneys, with approximately 86% of an administered dose recovered in the urine. The elimination half-life of Ropivacaine varies based on the route of administration, ranging from 1.8 hours (intravenous) to 4.2 hours (epidural).
The combination of its pharmacodynamic and pharmacokinetic properties makes Ropivacaine a versatile and effective local anesthetic, suitable for a wide range of clinical applications.
Clinical Efficacy and Safety
Efficacy in Different Types of Surgery
Ropivacaine has demonstrated significant efficacy in various surgical settings, including epidural, intrathecal, and peripheral nerve blocks. In epidural anesthesia, particularly for abdominal, gynecological, and orthopedic surgeries, Ropivacaine provides effective sensory blockade with minimal motor impairment, enhancing patient recovery and mobility. Its use in intrathecal anesthesia for lower limb surgeries has shown comparable onset and duration of sensory block to Bupivacaine, but with a shorter duration of motor block, which is advantageous for postoperative recovery.
Comparative studies highlight Ropivacaine’s effectiveness relative to other local anesthetics. For example, in labor pain management, Ropivacaine offers similar analgesic efficacy to Bupivacaine but with reduced motor blockade, allowing for better patient mobility during labor. Additionally, Ropivacaine has been favorably compared with Levobupivacaine in peripheral nerve blocks, providing adequate anesthesia for upper and lower limb surgeries with a faster onset and recovery profile.
Safety Profile
Ropivacaine’s safety profile is characterized by a lower incidence of cardiotoxicity and CNS toxicity compared to other local anesthetics like Bupivacaine. Its stereoselective properties and lower lipid solubility contribute to this improved safety, making it a preferred choice in clinical practice. The reduced potential for severe side effects is a significant advantage, particularly in high-risk populations.
Side effects associated with Ropivacaine are generally mild and include hypotension, nausea, vomiting, bradycardia, and headache. These are common with local anesthetics and are manageable with appropriate clinical interventions. The incidence of serious adverse events, such as CNS toxicity and cardiotoxicity, is significantly lower with Ropivacaine, enhancing its safety profile.
Guidelines for the safe use of Ropivacaine emphasize tailored dosing based on patient demographics, such as age, weight, and overall health. Special considerations are made for vulnerable populations, including children and pregnant women. In pediatric patients, lower doses are recommended to minimize the risk of toxicity, while in pregnant women, the drug is carefully administered to balance maternal and fetal safety.
In conclusion, Ropivacaine stands out for its efficacy in various surgical applications and its favorable safety profile, making it a versatile and reliable local anesthetic in clinical practice.
Advances and Research in Ropivacaine
Recent Studies and Findings
Ropivacaine has been the focus of numerous recent studies aimed at enhancing its clinical applications and formulations. One area of significant advancement is the development of sustained-release formulations. These new formulations aim to prolong the anesthetic effect of Ropivacaine, reducing the need for frequent dosing and improving patient comfort. For example, Ropivacaine-loaded liposomes and biodegradable microspheres have shown promise in preclinical and early clinical trials, providing extended pain relief with a single administration.
Another innovative approach involves the combination of Ropivacaine with other analgesic agents to enhance its efficacy and reduce side effects. The co-administration of Ropivacaine with adjuvants such as clonidine, dexmedetomidine, and opioids like fentanyl has been studied extensively. These combinations have been found to improve the quality of analgesia, reduce the required dose of Ropivacaine, and minimize motor block and systemic toxicity. Additionally, the use of Ropivacaine in multimodal analgesia protocols, where it is combined with other analgesic modalities, has shown to be effective in managing postoperative pain more efficiently.
Future Directions
Looking ahead, the potential new uses of Ropivacaine in pain management are expanding. Researchers are exploring its application in chronic pain conditions, such as neuropathic pain and cancer-related pain. Preliminary studies indicate that continuous peripheral nerve blocks and epidural infusions of Ropivacaine could offer significant relief in these challenging pain scenarios.
Ongoing clinical trials are crucial in determining the broader applicability of Ropivacaine. Trials investigating the optimal dosing strategies, long-term safety, and effectiveness of novel Ropivacaine formulations and delivery methods are currently underway. These trials aim to provide comprehensive data that could lead to the approval of new indications for Ropivacaine, enhancing its utility in both acute and chronic pain management.
Conclusion
Ropivacaine is a highly effective local anesthetic with a favorable safety profile, making it a preferred choice in various clinical settings. Advances in its formulation and delivery, combined with ongoing research, continue to expand its applications in pain management. Our company is dedicated to supporting the pharmaceutical industry by providing essential chemicals and compounds required for the synthesis, analysis, and optimization of Ropivacaine. As research progresses, Ropivacaine’s role in anesthesia and pain management is set to grow, offering better outcomes for patients.
During the production of Ropivacaine, various related chemicals and compounds play crucial roles. Impurities such as residual solvents and by-products from the synthesis process must be identified and quantified using advanced analytical techniques like high-performance liquid chromatography (HPLC) and gas chromatography (GC). Our company specializes in providing high-quality related chemicals, including impurities, building blocks, and isotope-labeled compounds essential for the synthesis and analysis of Ropivacaine.
The synthesis of Ropivacaine involves key chemical precursors and intermediates, such as 2,6-dimethylaniline and S(-)-1-propyl-2′,6′-pipecoloxylidide. Advances in synthetic methods, including enantioselective synthesis and improved catalytic processes, have optimized the production of Ropivacaine. These innovations increase yield and reduce impurity generation, making the manufacturing process more efficient and cost-effective. Our company offers a range of these critical building blocks to support the efficient synthesis of Ropivacaine.
Isotope-labeled Ropivacaine, where atoms in the molecule are replaced with their isotopes, is valuable in pharmacokinetic studies. These compounds help researchers track the drug’s absorption, distribution, metabolism, and excretion in the body with high precision. The use of isotope-labeled Ropivacaine enhances our understanding of its pharmacokinetics, facilitating the development of safer and more effective formulations. We supply high-purity isotope-labeled compounds to aid in these advanced pharmacokinetic studies.