Rewiring Fat: How β-Adrenergic Receptors Are Unlocking New Paths in Obesity and Metabolic Therapy
Abstract
Recent discoveries in adipose tissue biology have redefined fat from a passive energy reservoir to a dynamic endocrine organ with immense therapeutic potential. Central to this transformation is the evolving understanding of β-adrenergic receptors (βARs), particularly their role in activating lipolysis, thermogenesis, and browning of white fat. While rodent studies have shown promise in targeting β3-adrenergic receptors to combat obesity, translational hurdles in human physiology remain significant. New findings reveal that βAR signaling involves far more than the classical cAMP pathway—it engages intricate networks like ERK, p38 MAPK, and mTORC1. This expanded view opens the door to smarter, pathway-specific therapeutic strategies. By embracing the complexity of adipocyte signaling, researchers may unlock safer, more effective approaches to treating obesity, insulin resistance, and metabolic disease.
The Old Story Revisited — Why Fat Still Fascinates Scientists
For centuries, fat was seen as a passive energy store—an inert tissue that only accumulated when we overate and vanished when we exercised or starved. However, science now tells a dramatically different story. Far from being just a storage depot, adipose tissue is a metabolically active organ that plays central roles in energy balance, hormone secretion, and thermoregulation. In particular, the long-overlooked distinction between white adipose tissue (WAT) and brown adipose tissue (BAT) has reshaped our understanding of fat’s dynamic functions.
WAT primarily stores energy in the form of triglycerides, while BAT is specialized for nonshivering thermogenesis—the production of heat without muscle contractions. This thermogenic capability is driven by uncoupling protein 1 (UCP1) in the mitochondria of brown adipocytes. Historically, brown fat was thought to be present only in infants and small mammals. But modern imaging technologies, particularly PET scans, have confirmed the presence of active brown fat in adult humans as well, reigniting interest in its role in metabolic health and disease prevention.
At the center of this renewed attention are the β-adrenergic receptors (βARs)—cell surface proteins that respond to catecholamines like adrenaline and noradrenaline. These receptors regulate lipolysis (the breakdown of fat) and thermogenesis through cyclic AMP (cAMP)-mediated signaling. The discovery of the β3-adrenergic receptor (β3AR) in the 1980s added a new dimension to this field. Found predominantly in adipose tissue, β3AR has shown promising roles in activating brown fat and “browning” white fat in rodents, making it an attractive target for obesity therapies.
Despite decades of research, the complexity of βAR signaling continues to surprise researchers. The resurgence of interest in adipose biology is not just academic—it’s deeply relevant for tackling modern metabolic disorders such as obesity, type 2 diabetes, and cardiovascular disease. By reexamining this “old story” with new molecular tools and technologies, scientists are uncovering novel therapeutic pathways hidden in plain sight within our fat cells.
Meet the βAR Family — Three Receptors, One Complex Metabolism
At the heart of adipose tissue metabolism lies a trio of receptors that translate neural and hormonal cues into meaningful cellular responses: the β-adrenergic receptors (βARs). These G protein–coupled receptors—β1AR, β2AR, and β3AR—play a vital role in regulating fat breakdown and energy expenditure through cyclic AMP (cAMP) signaling. Though they share similar signaling mechanisms, each subtype operates with unique pharmacological and physiological properties, especially within adipose tissue.
β1AR and β2AR were the first to be discovered and are widely expressed in various tissues, including the heart, lungs, and adipocytes. In white and brown fat, their activation promotes lipolysis by stimulating cAMP production and activating protein kinase A (PKA), which in turn triggers hormone-sensitive lipase to mobilize stored triglycerides. These receptors also contribute to nonshivering thermogenesis, although to a lesser extent than β3AR.
The discovery of the β3-adrenergic receptor (β3AR) in the late 1980s was a turning point in adipocyte biology. Unlike its cousins, β3AR is predominantly expressed in adipose tissue—especially in brown and beige fat—and exhibits a unique ability to drive both lipolysis and thermogenesis. Its expression is notably high in rodents, making it a prime target in preclinical obesity research.
However, humans express much lower levels of β3AR in fat tissue, raising questions about its therapeutic viability. Despite this, selective β3AR agonists like CL316,243 in rodents and mirabegron in humans have shown potential in activating brown fat and enhancing energy expenditure under certain conditions. Interestingly, studies also suggest that β1AR and β2AR may play a more dominant role in human brown adipose metabolism, indicating species-specific functional hierarchies.
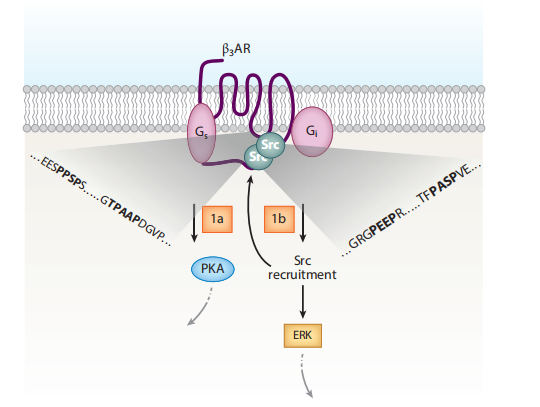
Figure 1. A unique dual signaling mechanism by β3-adrenergic receptor (β3AR).
Each βAR subtype, despite acting through cAMP, may engage distinct temporal and spatial signaling networks, interacting with different sets of proteins and pathways. This receptor diversity adds complexity to adipocyte responses and opens the door for selective therapeutic modulation, depending on the receptor target and the desired metabolic outcome.
Understanding this βAR signaling mosaic is essential as researchers and clinicians seek to harness adipose tissue for managing obesity, insulin resistance, and other metabolic disorders.
From Rodents to Humans — The Translational Roadblocks
Rodents have long served as the gold standard for metabolic research, offering insights into adipose biology and energy homeostasis. But as promising as these findings are in mice, translating them into effective therapies for humans has proven remarkably complex—especially in the case of β3-adrenergic receptors (β3AR) and brown adipose tissue (BAT).
In rodents, β3AR is highly expressed in both white and brown adipose tissue and serves as a powerful driver of lipolysis and nonshivering thermogenesis. Experimental compounds like CL316,243 produce profound reductions in fat mass and increases in energy expenditure in mouse models of obesity. However, humans express β3AR at much lower levels, and early trials with rodent-optimized drugs yielded underwhelming results in clinical settings.
For years, it was assumed that adult humans had minimal or no functional brown fat, further dampening enthusiasm for β3AR-targeted therapies. That changed dramatically in 2009, when PET-CT imaging studies using ^18F-fluorodeoxyglucose (^18F-FDG) confirmed metabolically active BAT in adult humans, particularly in the neck and supraclavicular regions. This discovery revived interest in the potential of brown fat as a therapeutic target for obesity and diabetes.
Pharmaceutical companies soon revisited β3AR agonists with renewed vigor. One such drug, mirabegron, originally approved for overactive bladder, was found to activate BAT and enhance glucose metabolism at higher doses. However, high-dose mirabegron also stimulates β1AR and β2AR, resulting in undesirable side effects like elevated heart rate and blood pressure.
Adding to the complexity, emerging evidence suggests that β1AR and β2AR may play a more prominent role than β3AR in human brown fat activation. This discrepancy underscores a key limitation: what works in rodents doesn’t always translate directly to human physiology. The pharmacological landscape in humans is shaped by differences in receptor distribution, tissue responsiveness, and safety profiles.
As research progresses, it’s becoming increasingly clear that species-specific receptor biology must be accounted for in drug development. The road from bench to bedside is rarely linear, and in the case of βARs and BAT, it demands precision, adaptability, and a deep understanding of human adipose signaling.
Beyond cAMP — New Signaling Pathways Rewrite the Playbook
For decades, scientists viewed β-adrenergic receptors (βARs) primarily through the lens of cyclic AMP (cAMP) signaling. When a βAR is activated by catecholamines like norepinephrine, it couples with Gαs proteins to increase intracellular cAMP, which then activates protein kinase A (PKA). This PKA cascade was thought to explain most, if not all, of the lipolytic and thermogenic effects in adipocytes.
But recent discoveries have shattered this one-pathway paradigm. It turns out that βAR signaling is far more versatile and multi-layered than previously understood—particularly in brown and beige fat. Among the three βAR subtypes, the β3-adrenergic receptor (β3AR) has emerged as uniquely capable of engaging alternative signaling routes that diverge from the classic cAMP-PKA axis.
One surprising finding is that β3AR can also couple to Gαi proteins, a property not commonly seen in other βARs. This dual coupling allows β3AR to trigger mitogen-activated protein kinase (MAPK)/ERK pathways independently of β-arrestins or cAMP production. These pathways influence not just metabolism but also gene expression and mitochondrial biogenesis, adding new depth to how adipocytes respond to adrenergic stimulation.
Even more unexpected is the discovery that βAR activation can influence mTORC1, a signaling complex traditionally associated with insulin and growth factor signaling. Studies now show that PKA can directly phosphorylate components of mTORC1, including mTOR and Raptor, enhancing thermogenic gene expression and supporting the “browning” of white adipose tissue. This means that catabolic and anabolic signaling cascades—once thought to be antagonistic—can be simultaneously activated by βARs in the context of thermogenesis.
Further downstream, kinases like p38 MAPK also play a critical role. Activation of p38 leads to phosphorylation of transcriptional coactivators like PGC1α, which drive the expression of UCP1 and other thermogenic genes. Together, these alternative pathways show that adipocyte signaling is more like an orchestra than a solo performance, with multiple kinases playing coordinated roles in fat metabolism.
These revelations not only enhance our understanding of adipose physiology but also open new avenues for drug development—ones that move beyond cAMP and toward more refined, receptor- and pathway-specific interventions.
What’s Next — Designing Smarter Therapies for Metabolic Disease
As obesity and metabolic disorders continue to challenge global healthcare systems, the search for safe and effective therapies has never been more urgent. Insights from adipose tissue research, particularly the evolving understanding of β-adrenergic receptor (βAR) signaling, offer new hope for developing smarter interventions. But success in this area will require more than simply targeting one receptor or pathway—it demands a systems-level approach to adipocyte biology.
Emerging research shows that adipocyte activation is not the result of a single signal but rather a convergence of multiple pathways. To engage in energy-expensive processes like nonshivering thermogenesis, adipocytes seem to require what author Sheila Collins metaphorically calls “multiple keys to unlock the safe deposit box.” These keys include βAR stimulation, cAMP signaling, p38 MAPK activation, mTORC1 engagement, and even input from natriuretic peptides via cGMP and PKG pathways.
This multi-key mechanism ensures tight regulation of thermogenesis, which is critical given its high energy demand. However, it also presents a translational opportunity: therapies that co-activate or selectively bias these pathways may offer better efficacy and fewer side effects than traditional stimulants. For example, selectively enhancing β3AR-mTORC1 coupling or targeting p38 MAPK signaling in specific fat depots could promote the browning of white adipose tissue without overstimulating the cardiovascular system.
Another exciting frontier is the concept of biased agonism—designing drugs that favor beneficial signaling arms (e.g., G protein vs. β-arrestin pathways) while minimizing undesirable effects. This precision pharmacology could allow us to tap into the metabolic benefits of brown and beige fat without the systemic burden of generalized adrenergic stimulation.
Yet, one of the biggest hurdles remains: species differences. What works in rodents often fails in humans due to differences in receptor expression, tissue distribution, and drug responsiveness. Future research must continue to leverage human-based models, including organoids, ex vivo adipose tissue, and advanced imaging, to close this translational gap.
In sum, the road to metabolic therapies lies not in reviving old paradigms, but in embracing the complexity of adipocyte signaling—and designing interventions that are as nuanced as the biology they aim to modulate.
References
Collins, S. (2022). β-Adrenergic receptors and adipose tissue metabolism: Evolution of an old story. Annual Review of Physiology, 84, 1–16.
https://doi.org/10.1146/annurev-physiol-060721-092939
Smith, R. E. (1964). Thermoregulatory and adaptive behavior of brown adipose tissue. Science, 146(3643), 1686–1689.
https://doi.org/10.1126/science.146.3643.1686
Cypess, A. M., Lehman, S., Williams, G., Tal, I., Rodman, D., Goldfine, A. B., … & Kolodny, G. M. (2009). Identification and importance of brown adipose tissue in adult humans. New England Journal of Medicine, 360(15), 1509–1517.
https://doi.org/10.1056/NEJMoa0810780
van Marken Lichtenbelt, W. D., Vanhommerig, J. W., Smulders, N. M., Drossaerts, J. M., Kemerink, G. J., Bouvy, N. D., … & Schrauwen, P. (2009). Cold-activated brown adipose tissue in healthy men. New England Journal of Medicine, 360(15), 1500–1508.
https://doi.org/10.1056/NEJMoa0808718
Emorine, L. J., Marullo, S., Briend-Sutren, M. M., Patey, G., Tate, K., & Strosberg, A. D. (1989). Molecular characterization of the human β3-adrenergic receptor. Science, 245(4922), 1118–1121.
https://doi.org/10.1126/science.2549643
Cero, C., Lea, H. J., Zhu, K. Y., Shamsi, F., Tseng, Y. H., & Cypess, A. M. (2021). β3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. JCI Insight, 6(3), e139160.
https://doi.org/10.1172/jci.insight.139160
Blondin, D. P., Nielsen, S., Kuipers, E. N., Severinsen, M. C., Jensen, V. H., Miard, S., … & Richard, D. (2020). Human brown adipocyte thermogenesis is driven by β2-AR stimulation. Cell Metabolism, 32(2), 287–300.e7.
https://doi.org/10.1016/j.cmet.2020.06.002
Cypess, A. M., Lehman, S., Williams, G., Tal, I., Rodman, D., Goldfine, A. B., … & Kolodny, G. M. (2009). Identification and importance of brown adipose tissue in adult humans. New England Journal of Medicine, 360(15), 1509–1517.
https://doi.org/10.1056/NEJMoa0810780
Finlin, B. S., Memetimin, H., Zhu, B., Confides, A. L., Vekaria, H. J., El Khouli, R. H., … & Kern, P. A. (2020). The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. Journal of Clinical Investigation, 130(5), 2319–2331.
https://doi.org/10.1172/JCI134892
Liu, D., Bordicchia, M., Zhang, C., Fang, H., Wei, W., Li, J. L., … & Collins, S. (2016). Activation of mTORC1 is essential for β-adrenergic stimulation of adipose browning. Journal of Clinical Investigation, 126(5), 1704–1716.
https://doi.org/10.1172/JCI83532
Ceddia, R. P., & Collins, S. (2020). A compendium of G-protein-coupled receptors and cyclic nucleotide regulation of adipose tissue metabolism and energy expenditure. Clinical Science, 134(4), 473–512.




