Rediscovery of the Prodrug Strategy: From Small Molecules to PROTAC Innovation
Abstract
Prodrugs are chemically modified therapeutics designed to improve drug properties such as solubility, bioavailability, and tissue selectivity. Traditionally applied to small molecules, the prodrug approach is now being extended to complex modalities like PROTACs, addressing their poor permeability and pharmacokinetics. This review outlines key prodrug strategies—enzyme activation, self-immolative linkers, redox/pH sensitivity—and highlights their roles in improving delivery and reducing toxicity. Special attention is given to “masked PROTACs,” which utilize cleavable moieties for controlled intracellular activation. Practical design considerations and evaluation methods are also discussed.
What Is a Prodrug?
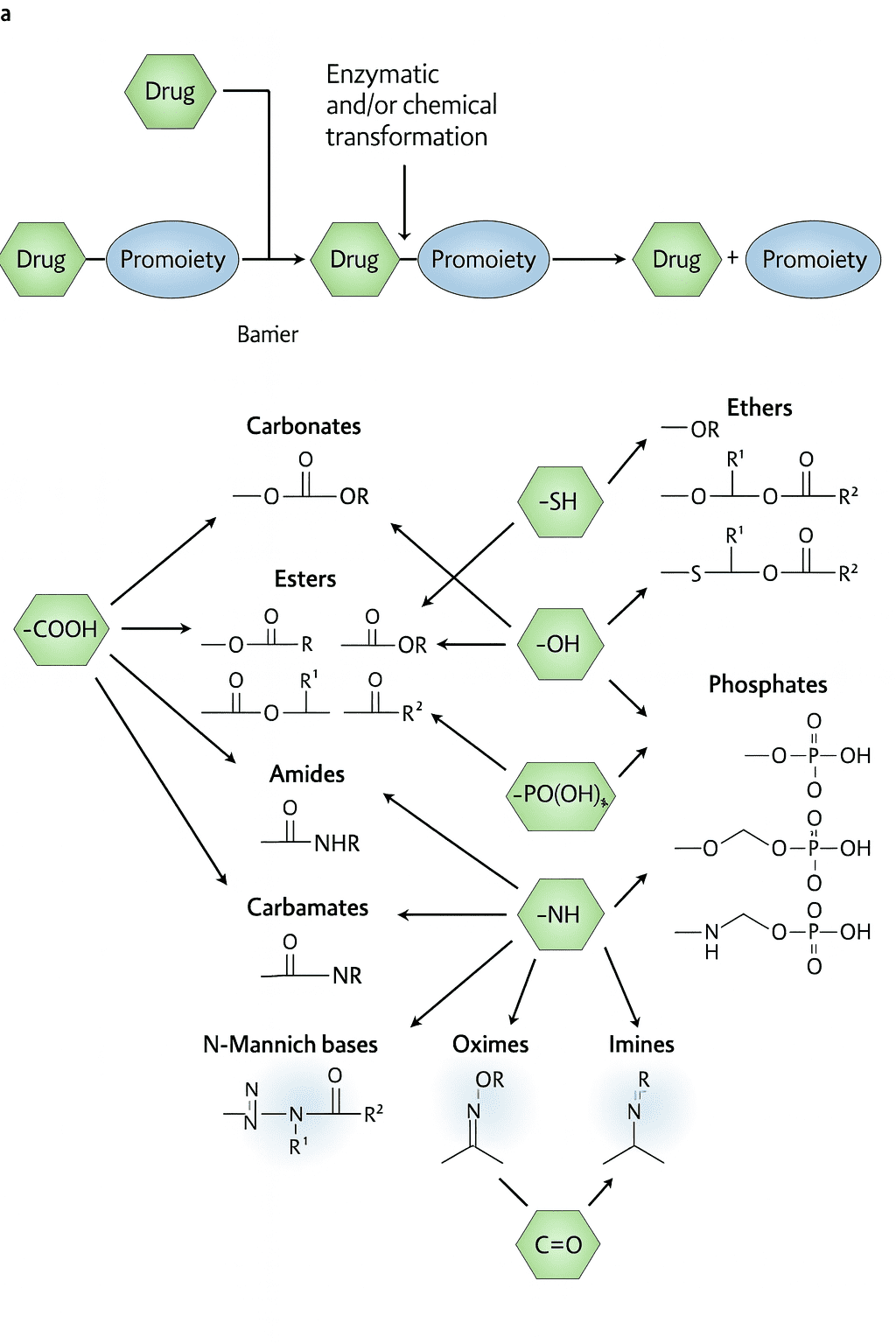
A prodrug is a drug design strategy that involves chemically modifying an active pharmaceutical ingredient (API) to improve its physicochemical properties or pharmacokinetics. The prodrug itself is inactive or less active and undergoes enzymatic or chemical transformation in the body to release the active parent drug. This approach addresses various drug development challenges such as poor solubility, low stability, insufficient bioavailability, off-target toxicity, and poor membrane permeability.
01 — Approved and Commonly Used Prodrugs
Enhancing Oral Absorption & Permeability
Esterification:
Enalapril → Enalaprilat, Irinotecan → SN-38, Valacyclovir (L-valine ester of Acyclovir), Valganciclovir (L-valine ester of Ganciclovir), Dabigatran etexilate.
Phosphate/Phosphonate Esters:
Oseltamivir phosphate, Etoposide phosphate, Fosphenytoin, Fosamprenavir.
Amino Acid/Dipeptide Conjugates:
Lisdexamfetamine (lysine prodrug for abuse deterrence and extended release).
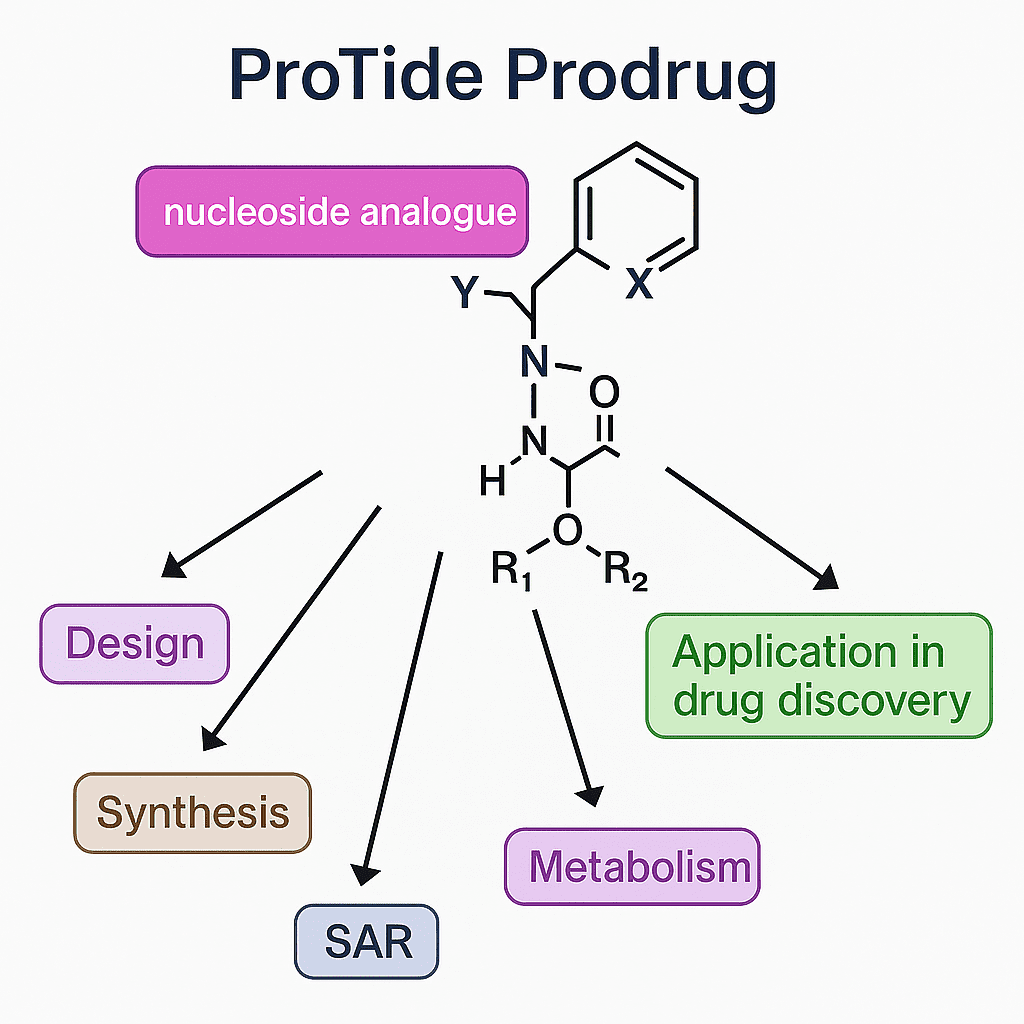
Nucleoside/Nucleotide Delivery (ProTide Strategy)
Phosphoramidate (ProTide) Prodrugs:
Sofosbuvir, Remdesivir, Tenofovir disoproxil fumarate (TDF), Tenofovir alafenamide (TAF).
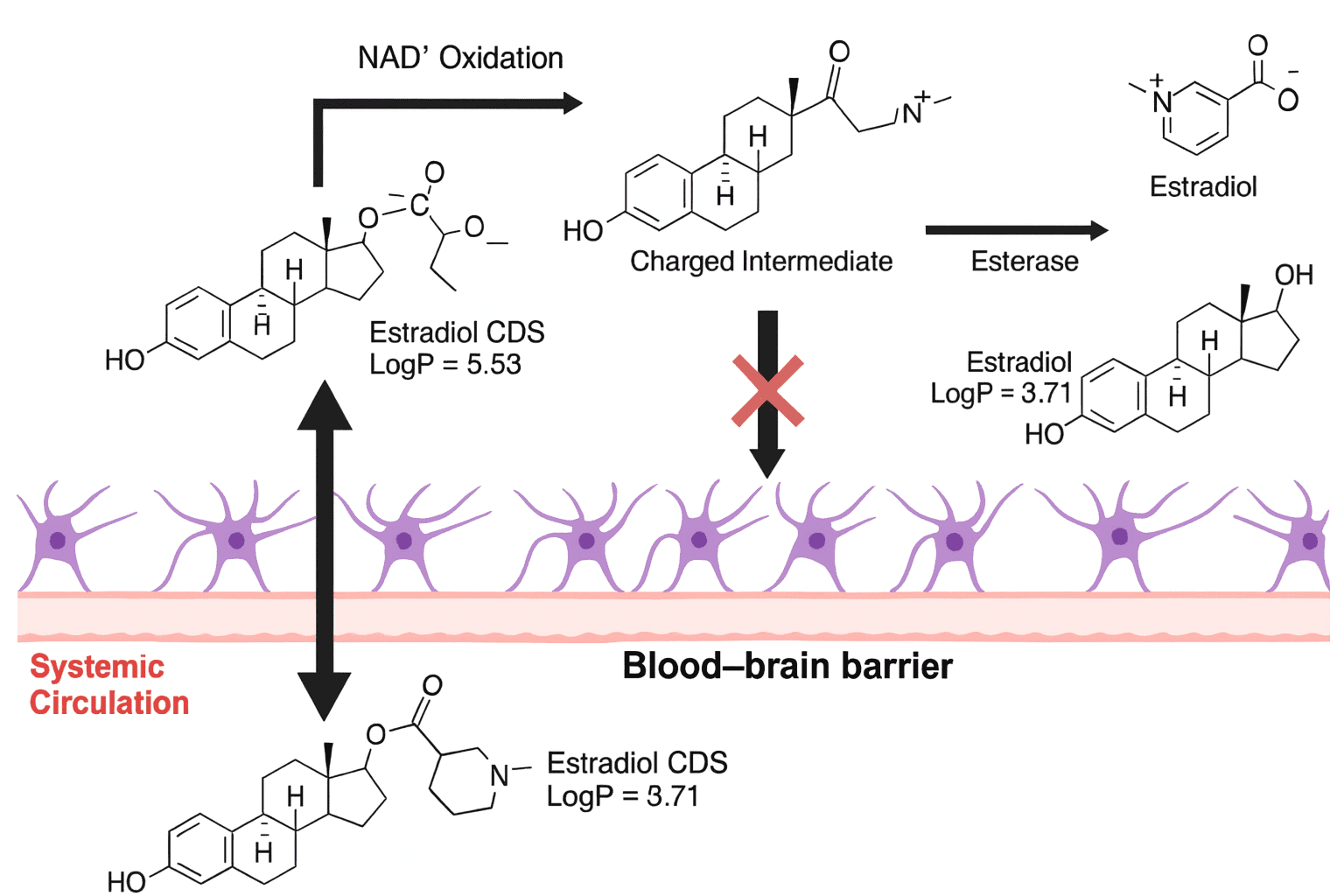
Blood–Brain Barrier (BBB) Penetration
Selective in vivo release of active metabolite:
Capecitabine → 5-FU, Clopidogrel/Prasugrel (CYP activation), Prednisone → Prednisolone, Sulfasalazine (colonic bacterial cleavage).
Neuroactive agents:
Levodopa (co-transported into the brain → dopamine), Codeine (partially a prodrug of morphine).
Roughly 10–15% of approved small-molecule drugs use a prodrug strategy in some form.
02 — How to Design a Prodrug (General Workflow)
Identify the Primary Limitation:
Poor permeability/absorption
High first-pass metabolism
Low solubility
Poor selectivity
Systemic toxicity
Targeted delivery required
Choose an Activation Mechanism (Trigger):
Enzymatic Activation:
Carboxyl esterases, carbonate/carbamate cleavage, dipeptides (e.g., Val-Cit for Cathepsin B), β-glucuronidase, alkaline phosphatase.
Chemical Activation:
pH-sensitive (acetal/hydrazone), redox-sensitive (disulfide, ROS-sensitive boronate), oxidative cleavage, photolysis (o-nitrobenzyl), self-immolative spacers (PABA, trimethyl-lock).
Tissue-Selective Activation:
Microbiota (azo bonds in colon), tumor microenvironment (hypoxia, ROS, low pH).
Choose the Promoiety:
Improve Solubility:
Phosphorylation, ionizable groups (quaternary ammonium).
Enhance Permeability:
Esterification, amino acid/dipeptide conjugation, lipid chains.
Targeting/Prolonged Circulation:
Glycosylation (GLUT, ASGPR), folate receptors, albumin binders (maleimide, fatty acids).
“Stability–Release” Balance:
Must be stable in plasma/GI tract yet release the active form at a rate matching the desired exposure profile.
In Vitro–In Vivo Correlation (IVIVC):
Account for species differences (e.g., esterase expression in human vs. rodent).
Build and calibrate PBPK and release models early.
Analytical and Biomarker Considerations:
Quantify parent drug/prodrug/promoiety metabolites.
Use mass spectrometry imaging, target occupancy, or pharmacodynamic biomarkers to confirm in vivo activation.
03 — Quick Reference: Common Prodrug Strategies
Functional Group Masking:
Carboxylic acid → ester, methyl/benzyl ether
Phenols → carbonates, phosphates
Amines → amides, carbamates
Phosphate/phosphonate → SATE, ProTide
Cleavable Linkers:
PABA (self-immolative), 1,6-elimination, Val-Cit, GFLG, disulfide, hydrazone (pH 5–6), o-nitrobenzyl (light), boronate (ROS)
Targeting and Gating:
Glycosylation (liver ASGPR, tumor glucose uptake), folate conjugation, RGD motifs
Macromolecular/polymer prodrugs (PEG, HA)
Environmental Response:
pH, enzymatic, ROS, reduction (GSH-rich cytosol/tumor), hypoxia, microbiota
04 — Key Takeaways for Small-Molecule Prodrug Applications
Improving Oral Bioavailability: Most common use (esters, amino acid esters, ProTide).
Unlocking Solubility: Phosphate prodrugs enable water-soluble injectable forms (e.g., etoposide phosphate).
Tissue/Cell Selectivity: Microbiota-cleaved, tumor enzyme/ROS/pH-triggered delivery to minimize systemic toxicity.
Controlled Release/Flattening Peaks: E.g., lisdexamfetamine reduces abuse potential and side effects.
Avoiding DDI/Genetic Polymorphism: Minimize reliance on CYP-dependent activation or develop companion diagnostics.
05 — Prodrug Strategies in PROTAC Development (“Pro-PROTACs” or “Masked PROTACs”)
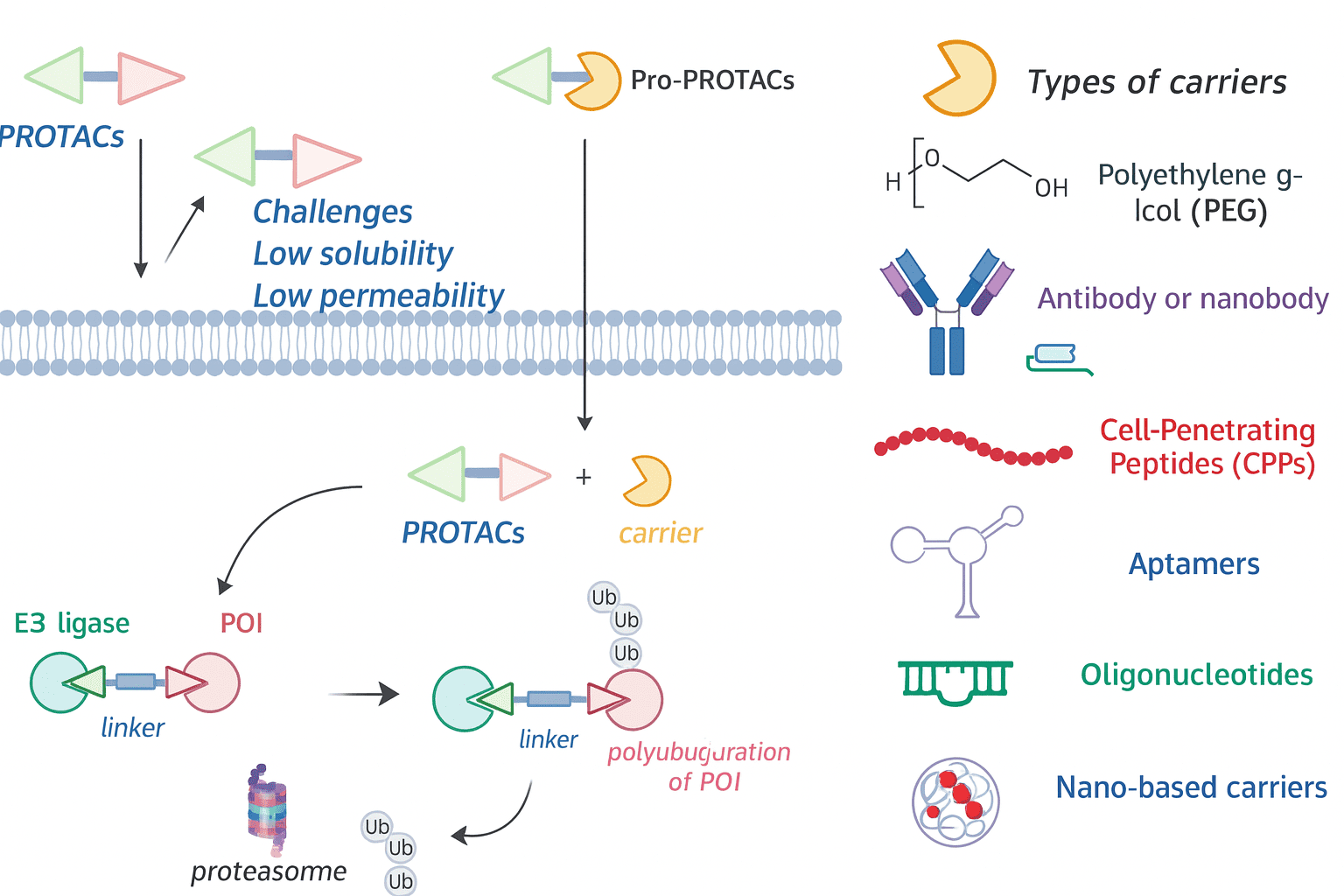
PROTACs are large, highly polar molecules with poor oral bioavailability and cell permeability. Prodrug strategies are essential in enabling their therapeutic use.
Common Strategies:
Esterase-Cleavable Lipophilic Masking
Methyl esters or carbonate esters on VHL ligand carboxyls or hydroxyls on linkers
Simple synthesis; balance plasma stability vs. rapid metabolism
Phosphate/Phosphonate Masking
Phosphate esters or ProTide-like phosphoramidates
Increases solubility for high-concentration injectable or local delivery
Activation by alkaline phosphatases inside cells
ROS/Reductive Activation
Disulfide linkers (GSH-sensitive), boronate esters (ROS-triggered self-immolation)
Suitable for tumor-selective release
Enzyme-Specific Release
Dipeptide linkers (Val-Cit, GFLG) cleaved by tumor-expressed CatB → 1,6-elimination
β-Galactosidase-activated PROTACs for targeting senescent cells
Controlled or Gated Activation (Non-classical Prodrugs)
PhotoPROTACs: light-activated (e.g., o-nitrobenzyl)
CLIPTACs: click chemistry in situ assembly from precursors
06 — Practical Considerations Often Overlooked
Safety of Promoieties: Evaluate exposure and accumulation of PABA, long-chain lipids, nitrobenzyl, quaternary ammonium.
Stereochemistry: Enantiomer-specific differences (e.g., ProTide, dipeptides) can affect enzyme recognition.
Formulation Synergy: Combine prodrug design with salt/cocrystal/amorphous formulation for additive benefits.
Analytical Methods: LC-MS tracking of prodrug → parent → metabolite → promoiety with full mass balance.
Companion Diagnostics: Genetic or biomarker-based selection when specific enzyme activation (e.g., CYP2C19) is required.
07 — Compact Yet Complete Design Checklist
Identify main development barriers (bioavailability, permeability, half-life, toxicity)
Select activation trigger (esterase, phosphatase, ROS, pH, light, microbiota)
Choose promoiety (solubility, permeability, targeting)
Design self-immolative linker (if needed)
Build in vitro release/stability matrix (plasma, S9, pH, GSH, ROS, species comparison)
Conduct early DMPK studies (IV/oral exposure, tissue distribution, clearance)
Assess safety (promoiety toxicity, hERG, genotoxicity, DDI)
Validate in vivo activation (mass spectrometry imaging, occupancy, degradation curves)
Evaluate cost and synthetic feasibility (1–2 step deprotection, scale-up stability)
References
Rautio, J. et al. (2018). The expanding role of prodrugs in contemporary drug design and development. Nat Rev Drug Discov, 17(8), 559–587.
https://www.nature.com/articles/nrd.2018.46
Rautio, J. et al. (2008). Prodrugs: Design and clinical applications. Nat Rev Drug Discov, 7(3), 255–270.
https://www.nature.com/articles/nrd2468
Mehellou, Y. et al. (2018). The ProTide prodrug technology: From the concept to the clinic. J Med Chem, 61(6), 2211–2226.
https://pubs.acs.org/doi/abs/10.1021/acs.jmedchem.7b00734
Huttunen, K. M. et al. (2011). Prodrugs—from serendipity to rational design. Pharmacol Rev, 63(3), 750–771.
https://www.sciencedirect.com/science/article/abs/pii/S0031699724009281
Stella, V. J. & Nti-Addae, K. W. (2007). Prodrug strategies to overcome poor water solubility. Adv Drug Deliv Rev, 59(7), 677–694.
https://www.sciencedirect.com/science/article/abs/pii/S0169409X07000865
Lai, A. C. & Crews, C. M. (2017). Induced protein degradation: An emerging drug discovery paradigm. Nat Rev Drug Discov, 16(2), 101–114.
https://www.nature.com/articles/nrd.2016.211
Békés, M. et al. (2022). PROTAC targeted protein degraders: The past is prologue. Nat Rev Drug Discov, 21(3), 181–200.