Peptide-Based Cancer Inhibitors: The Future of Precision Oncology
Abstract
Peptide-based inhibitors are rapidly emerging as a powerful and precise class of therapeutics in cancer treatment. Unlike traditional small-molecule drugs, peptides offer high specificity, low toxicity, and the ability to disrupt protein–protein interactions—making them ideal for targeting oncogenic pathways. This blog explores the current landscape of peptide inhibitors, including their molecular targets, delivery innovations, and clinical applications. It also highlights future directions, such as AI-assisted peptide design, tumor microenvironment-responsive systems, and multifunctional peptides for combined therapeutic and diagnostic use. As advances in biotechnology and personalized medicine converge, peptide therapeutics are poised to redefine the future of precision oncology.
Peptide-Based Cancer Inhibitors: Why They’re the New Frontier in Targeted Therapy
In the battle against cancer, researchers are constantly looking for therapies that are more precise, effective, and less toxic. Traditional treatment approaches—like chemotherapy and small-molecule inhibitors—have made great strides, but they also come with significant drawbacks, including off-target toxicity, drug resistance, and limited selectivity. In recent years, peptide-based therapeutics have emerged as a powerful and promising alternative, offering new hope in the fight against tumors.
Peptides are short chains of amino acids that can be engineered to mimic or disrupt biological functions with high precision. Unlike conventional drugs, peptides can specifically target protein–protein interactions (PPIs), which are often critical for cancer cell survival but notoriously difficult to drug. Their small size, tunable structure, and low immunogenicity make them attractive tools for targeting previously “undruggable” pathways in cancer biology (Muttenthaler et al., 2021).
Moreover, peptides offer high specificity with minimal systemic toxicity, making them ideal for selective targeting of cancer cells while sparing healthy tissue. This translates into better safety profiles and improved patient outcomes, particularly in hard-to-treat tumors where precision is key (Katsoris et al., 2023).
Recent advances in peptide synthesis, modification, and delivery systems have also resolved many of the historical limitations of peptide drugs—such as poor stability or rapid degradation. Techniques like PEGylation, cyclization, and D-amino acid substitution enhance peptide resistance to proteolysis, while nanoparticles, liposomes, and cell-penetrating peptides improve delivery to tumor sites (Adessi & Soto, 2002; Fosgerau & Hoffmann, 2015).
With several peptide-based drugs already approved and many more in clinical development, the field is poised for rapid expansion. Peptides are no longer just tools for basic research—they are becoming frontline candidates in targeted cancer therapy, capable of delivering both therapeutic and diagnostic functions.
This blog series explores the types of peptide inhibitors, their mechanisms, delivery innovations, and the future directions shaping this fast-evolving field.
Types of Peptide Inhibitors — Blocking Cancer at the Molecular Level
One of the most exciting features of peptide-based cancer therapeutics is their versatility in targeting different molecular mechanisms. These peptides are not “one-size-fits-all”—they are precision tools engineered to disrupt specific signaling events that drive cancer progression, survival, and metastasis.
Peptides Targeting Growth Factor Receptors
Many tumors rely on growth factor signaling to thrive. Peptides such as GE11 specifically bind to EGFR (epidermal growth factor receptor), inhibiting downstream signaling involved in cell proliferation and angiogenesis. Unlike monoclonal antibodies, these peptides offer better tissue penetration and reduced immunogenicity (Katsoris et al., 2023).
Peptides Disrupting Protein–Protein Interactions
Protein–protein interactions (PPIs) like MDM2–p53 and BCL-2–BAX are crucial for cancer cell survival. Peptides that block these interactions can restore apoptotic pathways or reactivate tumor suppressors like p53. Others, like RAS-targeting peptides, interfere with oncogenic signaling in the MAPK and PI3K pathways—major drivers of aggressive tumor growth (Tan et al., 2016).
Peptides Affecting Cell Cycle and Apoptosis
Peptides that inhibit cyclin-dependent kinases (CDKs) or activate caspases can halt uncontrolled cell division or promote apoptosis. Survivin-targeting peptides, for example, destabilize anti-apoptotic signaling in cancer cells.
Peptides Inhibiting Metastasis and EMT
Cancer metastasis is a major cause of treatment failure. Peptides that block integrins, MMPs, or EMT transcription factors like Snail and Twist help suppress tumor invasion and migration.
Each class of peptide inhibitor contributes to a multi-angle attack on cancer, offering a modular toolkit for designing highly targeted therapies.
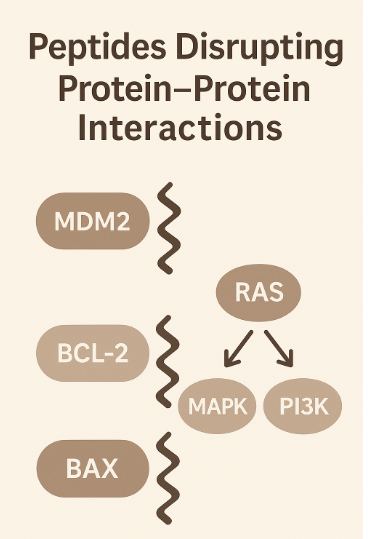
Figure 1. Targeting Tumor Pathways
Delivery Challenges and Smart Solutions
While peptide-based inhibitors offer enormous therapeutic potential, their clinical success depends on overcoming key delivery barriers. These include enzymatic degradation, poor membrane permeability, and short systemic half-life—all of which can limit their bioavailability and effectiveness in cancer treatment.
Chemical Modifications for Stability
To improve stability, peptides are often chemically modified:
Cyclization helps protect against enzymatic degradation.
Substitution of D-amino acids or N-methylated residues increases resistance to proteolysis.
PEGylation—attaching polyethylene glycol chains—extends half-life and reduces renal clearance (Fosgerau & Hoffmann, 2015).
Advanced Delivery Systems
Efficient delivery is critical for peptides to reach and accumulate in tumors:
Nanoparticles and liposomes can encapsulate peptides, shielding them from degradation and enabling passive or active targeting to tumors.
Exosomes, the body’s natural delivery vesicles, offer low immunogenicity and can carry therapeutic peptides across biological barriers.
Cell-penetrating peptides (CPPs) such as TAT or penetratin facilitate transport of cargo across cellular membranes, improving intracellular bioavailability (Guidotti et al., 2017).
Tumor-Targeting Strategies
Peptides can be engineered with ligands that recognize tumor-specific receptors, enabling precision delivery. These smart systems increase drug accumulation at tumor sites while minimizing damage to healthy tissues.
By integrating chemistry and nanotechnology, researchers are rapidly transforming peptides into clinically viable cancer therapeutics with improved delivery profiles.
Clinical Progress and Real-World Applications of Peptide Cancer Inhibitors
Peptide-based inhibitors are no longer just theoretical tools or laboratory curiosities—they are actively shaping the future of cancer therapy. Several peptides have already transitioned from bench to bedside, demonstrating both safety and efficacy in clinical settings. These advancements not only validate peptide therapeutics but also pave the way for broader applications in precision oncology.
FDA-Approved Peptide Drugs in Oncology
One of the most prominent examples is Bortezomib, a dipeptide boronic acid approved for the treatment of multiple myeloma and mantle cell lymphoma. Bortezomib works by inhibiting the proteasome, disrupting protein homeostasis and triggering cancer cell apoptosis. Its success has highlighted how peptide-based molecules can be highly potent and clinically viable (Fosgerau & Hoffmann, 2015).
In the hormone-related cancer space, LHRH analogs such as Leuprolide and Goserelin are widely used in the management of prostate and breast cancers. These peptides suppress gonadotropin release, effectively reducing hormone levels that drive tumor growth. Their long-standing clinical use underscores the stability and tolerability achievable with proper peptide formulation.
Peptides in Clinical Trials
Beyond approved therapies, numerous peptide inhibitors are in various stages of clinical development. These include:
Peptides that target integrins to prevent tumor metastasis.
EGFR-binding peptides like GE11, under investigation as tumor-targeting ligands for drug-conjugates and imaging agents.
MDM2–p53 interaction inhibitors, designed to reactivate tumor suppressor functions.
Many of these candidates are showing promising results in phase I and II trials, particularly in solid tumors like lung, colorectal, and pancreatic cancers, where traditional treatments often fail.
Theranostic Applications
Interestingly, peptides are also being explored for dual therapeutic and diagnostic (theranostic) roles. For example, tumor-targeting peptides can be labeled with radioisotopes or fluorescent dyes, allowing simultaneous tumor visualization and treatment—a major advantage in monitoring therapeutic efficacy.
As the field advances, peptide drugs are likely to become essential components in personalized cancer treatment, either as monotherapies or in combination with chemotherapy, immunotherapy, or targeted agents.
Future Directions: Designing the Next Generation of Peptide Cancer Drugs
As peptide therapeutics continue to gain momentum in oncology, the future of this field lies in intelligent design, multifunctionality, and personalization. With a growing understanding of tumor biology and technological advances in drug delivery and molecular modeling, researchers are now developing next-generation peptides that go far beyond simple inhibitors.
AI and In Silico Design
Modern drug discovery increasingly leverages artificial intelligence (AI) and computational modeling to design novel peptide sequences with high binding affinity, improved stability, and optimal pharmacokinetic profiles. AI tools can rapidly screen libraries of virtual peptides and predict their behavior in biological systems—reducing development time and cost (Yu et al., 2022). These approaches make it possible to tailor peptides for specific cancer types, receptors, and even patient genotypes.
Multifunctional Peptides
Researchers are moving toward multifunctional peptide platforms that can deliver combined effects, such as:
Blocking oncogenic signaling,
Inducing apoptosis,
Delivering chemotherapeutics or nucleic acids, and
Acting as imaging agents for tumor diagnostics.
Such “theranostic” peptides offer a dual advantage—targeted therapy and real-time monitoring—thereby improving treatment precision and responsiveness.
Tumor Microenvironment (TME)-Responsive Systems
Next-gen peptides are also being engineered to respond to the tumor microenvironment. For example, peptides can be designed to activate only under acidic pH, high enzyme levels, or oxidative stress—conditions commonly found in tumors. These stimuli-sensitive systems ensure that peptides remain inactive in healthy tissue and become active only within the tumor, minimizing side effects and maximizing efficacy (Katsoris et al., 2023).
Personalized Peptide Vaccines
Another emerging trend is the development of personalized peptide-based cancer vaccines. These vaccines are designed from tumor-specific antigens identified through genomic sequencing and can stimulate the patient’s immune system to recognize and destroy cancer cells. Early trials in melanoma and glioblastoma show encouraging immune responses and durable clinical outcomes.
Conclusion
The future of peptide cancer therapeutics is not just about inhibition—it’s about precision, adaptability, and synergy. With advances in AI, smart delivery systems, and tumor-specific targeting, peptide drugs are poised to lead the next wave of personalized oncology.
References
Adessi, C., & Soto, C. (2002). Converting a peptide into a drug: Strategies to improve stability and bioavailability. Current Medicinal Chemistry, 9(9), 963–978.
https://doi.org/10.2174/0929867024606755
Fosgerau, K., & Hoffmann, T. (2015). Peptide therapeutics: Current status and future directions. Drug Discovery Today, 20(1), 122–128.
https://doi.org/10.1016/j.drudis.2014.10.003
Katsoris, P., Katsoulieris, E. N., Kalemia, I. A., & Lamari, F. N. (2023). Peptide-Based Inhibitors as Potential Therapeutic Agents for Cancer: Current Insights and Future Perspectives. International Journal of Molecular Sciences, 24(21), 17600.
https://doi.org/10.3390/ijms242117600
Muttenthaler, M., King, G. F., Adams, D. J., & Alewood, P. F. (2021). Trends in peptide drug discovery. Nature Reviews Drug Discovery, 20(4), 309–325.
https://doi.org/10.1038/s41573-020-00135-8
Tan, M. L., Choong, P. F., & Dass, C. R. (2016). Peptide-based drug delivery systems for cancer therapeutics. Journal of Drug Targeting, 24(9), 763–773.
https://doi.org/10.3109/1061186X.2015.1131173
Fosgerau, K., & Hoffmann, T. (2015). Peptide therapeutics: Current status and future directions. Drug Discovery Today, 20(1), 122–128.
https://doi.org/10.1016/j.drudis.2014.10.003
Guidotti, G., Brambilla, L., & Rossi, D. (2017). Cell-penetrating peptides: From basic research to clinics. Trends in Pharmacological Sciences, 38(4), 406–424.
https://doi.org/10.1016/j.tips.2017.01.003
Yu, Y., Zhang, L., Xu, X., Wang, Q., & Zhang, C. (2022). Artificial intelligence in peptide drug discovery. Drug Discovery Today, 27(5), 1360–1372.




