PEI Transfection Reagents: Mechanisms, Optimization, and Applications in Modern Gene Delivery
Abstract
Polyethylenimine (PEI) is one of the most widely used non-viral transfection reagents for gene delivery in mammalian cells. Its unique cationic structure enables efficient condensation of nucleic acids, cellular uptake, and gene expression through mechanisms such as the debated “proton sponge” effect. Both linear and branched PEI formulations have been developed, with linear derivatives, such as PEI MAX and PEIpro, showing superior performance in transient gene expression (TGE) systems like HEK293 and CHO. This blog reviews the chemistry, mechanism of action, and structural variations of PEI; highlights optimization parameters for improved transfection efficiency; and addresses key considerations for scaling up from bench to bioreactor. Current challenges, including cytotoxicity and variability, are discussed alongside emerging trends in degradable derivatives and GMP-grade reagents that aim to improve safety and reproducibility. Together, PEI remains a robust and versatile platform for basic research, bioproduction, and clinical development.
PEI Transfection Reagents: A Practical, Modern Guide
Polyethylenimine (PEI) has been the workhorse polymer for DNA delivery in mammalian cells for nearly three decades. It’s cheap, scalable, robust across many cell types, and—when optimized—can rival premium lipids for transient gene expression (TGE) in HEK293 and CHO. If you’ve ever made antibodies by TGE or produced viral vectors at scale, chances are PEI was involved. This post distills the essentials: chemistry and mechanism, linear vs. branched and common brands, how to tune conditions that actually move the needle, pitfalls, scale-up, and the latest thinking on safety and clinical trajectories.
What PEI is and why it works
PEI is a cationic polymer composed of repeating ethylenimine units that present a high density of protonable amines. Because “every third atom” is an amino nitrogen, PEI condenses nucleic acids into nanoscale polyplexes and interacts strongly with the anionic glycocalyx and membranes to promote uptake. The classic study by Boussif and colleagues (1995) put PEI on the map as a versatile nonviral vector for cells in culture and even in vivo, with efficiencies matching or surpassing contemporaneous lipopolyamines.
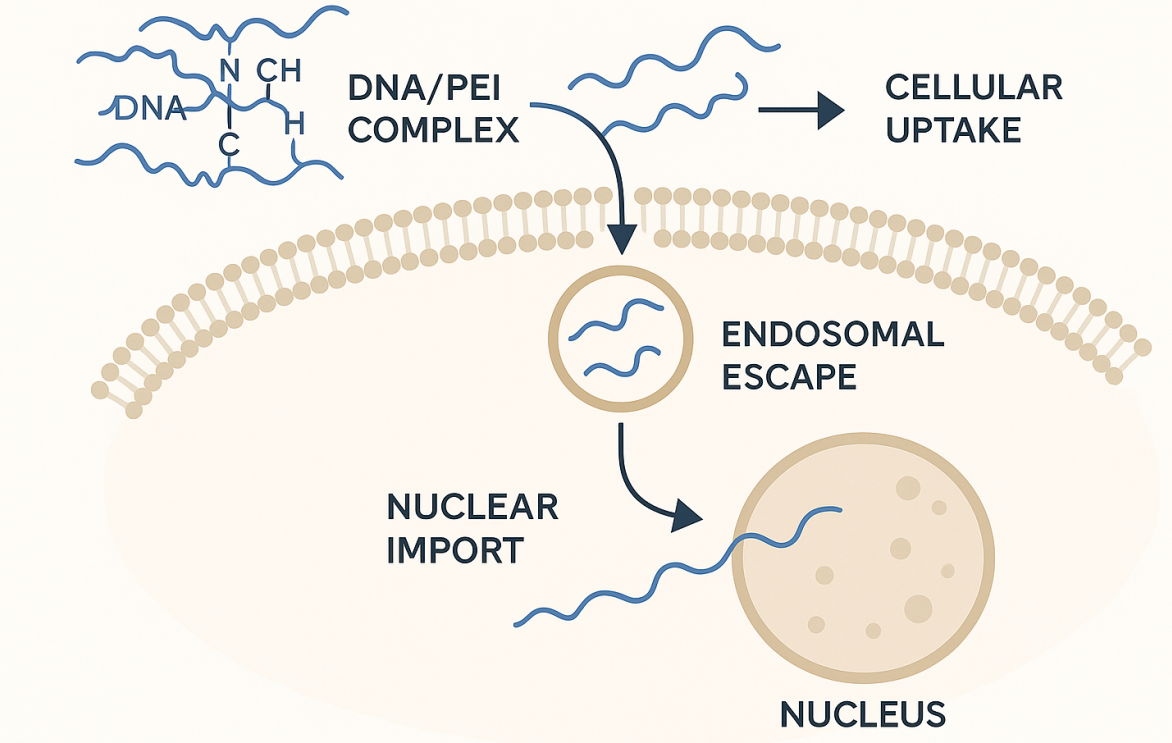
FIG.1 Mechanism of Action of PEI-Mediated Gene Delivery
Endosomal escape: proton sponge—and beyond
A widely cited explanation for PEI’s activity is the “proton sponge” effect: its buffering capacity drives proton (and counter-ion) influx into endosomes, increasing osmotic pressure and aiding escape. The hypothesis is influential but debated; quantitative lysosomal pH measurements indicate PEI may not measurably alkalinize lysosomes, suggesting additional or alternative mechanisms contribute to escape. The take-home for practitioners is pragmatic: PEI works, but the precise biophysics is more nuanced than a single mechanism.
Linear vs. branched PEI—and what’s in the bottle
Two structural families dominate: branched PEI (bPEI) (e.g., 25 kDa) and linear PEI (lPEI) (e.g., 25 or 40 kDa). In HEK293/CHO TGE, modern linear formulations—particularly highly de-acylated, well-defined polymers—tend to give higher expression with lower variability than legacy bPEI. Head-to-head comparisons show that de-acylated linear PEIs (e.g., PEI MAX ~40 kDa; PEIpro) often outperform partially acylated LPEI 25 kDa for secreted protein expression, though toxicity profiles and optimal DNA:PEI ratios differ.
What,s commonly used:
LPEI 25 kDa (classic lab-made solutions).
PEI MAX 40 kDa (linear)—de-acylated, widely used from plates to bioreactors, and often a “gold standard” for TGE cost-effectiveness.
PEIpro (linear)—chemically defined, offered in HQ/GMP grades for virus production and process continuity.
jetPEI (linear)—optimized, standardized lots; extensively protocolized for in vitro and in vivo use.
How to optimize PEI transfection (what actually matters)
Dozens of knobs exist, but not all are equal. These are the ones that consistently change outcomes.
1) DNA quality and design
Endotoxin-free, supercoiled plasmid DNA with a strong promoter and optimized cassette is table stakes. Minimize bacterial backbone burden and CpG content when possible; these reduce innate responses and stabilize expression (particularly for longer runs).
2) Cell health, density, and medium
High viability (≥95%), exponential growth phase, and the right viable cell density (VCD) at transfection time are critical. For suspension HEK293/CHO, many published protocols target low-to-mid 10^6 cells/mL; optimized high-density methods push higher with appropriate feeding/aeration. Manufacturer protocols and bioprocess notes increasingly express DNA and PEI per million cells to keep dosing consistent across scales.
3) PEI:DNA ratio and complexation
PEI polyplex formation is sensitive to charge ratio (commonly expressed as mass ratio or N/P). The best ratio depends on polymer chemistry, cell type, and medium. Studies show that mixing order, buffer (salt, pH), and complexation time can swing efficiency and reproducibility. A good starting grid for many linear PEIs is DNA:PEI (w:w) around 1:2–1:3; then titrate. For bPEI 25 kDa with primary cells, successful protocols exist but demand careful optimization of buffer and timing.
4) Media effects and additives
Serum and proteins can inhibit complexation and uptake, so many workflows form complexes in protein-free medium (e.g., Opti-MEM, PBS, or bespoke buffers) and add to cultures grown in serum-free systems. For TGE, post-transfection supplements (feeds, sodium butyrate or valproic acid in some CHO processes) can significantly boost titers—at the cost of higher stress and sometimes lower viability. (VPA has documented titer benefits in CHO DG44.)
5) Temperature, timing, and post-transfection handling
Polyplex contact time (exposure window) and temperature shifts (e.g., to 32–34 °C after transfection) can increase yields for secreted proteins. Harvest windows for maximal product often trail peak cell-specific productivity; don’t chase early fluorescent readouts that miss later production dynamics.
6) Lot-to-lot and mixing artifacts
Even with commercial, defined PEIs, lot-to-lot differences can exist. Gentle but thorough mixing, avoiding bubbles/foaming, and respecting the complexation time your reagent expects all improve run-to-run consistency. Comparative studies noted differences in polydispersity and DNA affinity among LPEI 25 k, PEI MAX 40 k, and PEIpro that correlate with performance and toxicity—reasons to qualify lots for critical processes.
Bench-top protocols: a quick blueprint
Below is a generic outline you can adapt to your PEI brand and cells; always check the manufacturer’s protocol for brand-specific concentrations.
For HEK293 suspension (1 L example, per PEIpro protocol)
Measure VCD (e.g., 2 × 10^6 cells/mL).
Dilute 2 mg DNA in ~50 mL protein-free medium.
Dilute your PEI to the recommended volume per million cells (per vendor table), then combine with DNA, gentle mix, incubate as specified (e.g., ~10–20 min).
Add polyplexes to culture while agitating.
Feed and, if relevant, apply temperature shift or additives per your expression strategy.
Adherent primary cells (branched PEI 25 kDa)
Optimize in small plates: vary salt, volume, incubation time, and PEI:DNA ratio; some protocols complete in ~2 days from complexation to readout.
For in-vitro DNA delivery across many adherent lines, standardized jetPEI procedures can be a rapid way to get “first light” and then refine.
Scaling up: from plates to bioreactors
PEI is particularly attractive for TGE and viral vector production because it scales linearly with cells—and it’s inexpensive. Industry posters and protocols demonstrate PEI-driven processes across plates, CellSTACKs, WAVE/rockers, and stirred tanks. Corning outlines practical steps for CellSTACK transfections with PEIpro; Polyplus provides HQ/GMP continuity for clinical manufacturing, and many groups have documented high-density HEK293E/CHO methods that double transient titers versus conventional setups.
A few scale-up pointers:
Dose by cells, not volume. Express DNA and PEI per 10^6 cells to maintain equivalence across culture volumes.
Mass transfer matters. Agitation, gas transfer, and CO₂ control change polyplex collision rates and uptake; re-optimize exposure times at new scales.
Feeding strategies (glucose, peptones, lipids) may extend production windows; track osmolality and lactate to avoid stress-induced crashes.
Analytics: don’t rely solely on early reporter readouts. Follow product titer, specific productivity (qP), and quality attributes over time.
Troubleshooting: common failure modes and fixes
Low expression, good viability
Likely under-dosed or complexes too “soft.” Increase PEI slightly or re-form complexes at higher concentration/lower volume; check DNA integrity and endotoxin.
High toxicity, low expression
Too much PEI or harsh polyplexes. Reduce PEI; extend complexation time; switch to a de-acylated linear PEI or a different PEI brand; ensure medium is compatible.
Great GFP, poor secreted protein
Optimize post-transfection feeds, temperature shift, and harvest window. Some conditions boost early transcription/translation without maximizing secretion.
Run-to-run variability
Standardize everything: DNA prep lot, PEI lot, water/buffer source, complexation mixer and time, cell age and split schedule. Consider defined linear PEIs with tighter specs.
Safety and handling
PEI is irritant and cytotoxic at higher doses. Always prepare stocks in appropriate buffers (many vendors supply ready-to-use solutions), filter sterilize if you make your own, and store as recommended. For in vivo work (e.g., lung delivery), dedicated formulations like in vivo-jetPEI come with route-specific guidance; do not extrapolate cell-culture doses to animals without a validated protocol.
Where the field is going
Two main vectors of progress are clear:
Better-defined linear PEIs and GMP paths. Reagents like PEIpro (HQ/GMP) and standardized jetPEI lots reduce variability and support translation to clinical-grade vector manufacturing.
New chemistries to ease toxicity and improve delivery. Reviews over the last few years catalog degradable PEI derivatives, ligand-targeted constructs, and hybrid materials that aim to keep PEI’s potency while improving safety. Clinical translation remains limited relative to bench popularity, but the pipeline is active and increasingly sophisticated.
A quick decision guide (TL;DR)
First pass in HEK293/CHO TGE? Start with a modern linear PEI (PEI MAX or PEIpro) and the vendor’s baseline protocol. Then titrate DNA:PEI and exposure time at your working VCD.
Primary/adherent cells? bPEI 25 kDa can work, but optimization of complexation buffer and timing is essential; consider standardized kits (jetPEI) to get started.
Scaling to CellSTACKs/bioreactors or making virus? Use cell-normalized dosing, defined linear PEIs, and scale-appropriate mass transfer; follow established CellSTACK/virus production protocols.
Seeing inconsistencies? Audit lot quality, complexation steps, and media interactions; remember that polymer chemistry (de-acylation, polydispersity) strongly impacts outcomes.
References
Boussif, O., Lezoualc’h, F., Zanta, M. A., Mergny, M. D., Scherman, D., Demeneix, B., & Behr, J.-P. (1995). A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proceedings of the National Academy of Sciences of the USA, 92(16), 7297–7301.
https://doi.org/10.1073/pnas.92.16.7297
Benjaminsen, R. V., Mattebjerg, M. A., Henriksen, J. R., Moghimi, S. M., & Andresen, T. L. (2013). The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Molecular Therapy, 21(1), 149–157.
https://doi.org/10.1038/mt.2012.185
Delafosse, A., Xu, P., Durocher, Y., & Hincapie, M. (2016). Comparative study of polyethylenimines for transient gene expression in mammalian HEK293 and CHO cells. Journal of Biotechnology, 227, 103–111.
https://doi.org/10.1016/j.jbiotec.2016.04.028
Delafosse, A., et al. (2016). Data on physicochemical properties of LPEI 25 kDa, PEI “Max” 40 kDa and PEIpro™. Data in Brief, 8, 456–460.
https://doi.org/10.1016/j.dib.2016.05.066
Hsu, C. Y. M., & Uludağ, H. (2012). A simple and rapid nonviral approach to efficiently transfect primary tissue-derived cells using polyethylenimine. Nature Protocols, 7(5), 935–945.
https://doi.org/10.1038/nprot.2012.038
Schenk, M., Parhizkar, E., Detampel, P., Dehshahri, A., & Huwyler, J. (2023). Polyethylenimine (PEI) in gene therapy: Current status and clinical prospects. Journal of Controlled Release, 355, 244–266.