Peficitinib Hydrobromide: A New Oral JAK Inhibitor for Rheumatoid Arthritis Treatment
Abstract
Peficitinib Hydrobromide is a Janus kinase (JAK) inhibitor developed to treat moderate-to-severe rheumatoid arthritis, particularly in patients unresponsive to conventional DMARDs. It targets JAK1, JAK2, JAK3, and TYK2, reducing inflammation through immune pathway modulation. Clinical trials have shown that peficitinib provides effective symptom control and disease remission, with a manageable safety profile over long-term use. Its oral administration offers convenience and improved adherence compared to injectable alternatives. While currently approved in select Asian markets, ongoing research continues to explore its global potential and application in other autoimmune diseases.
Introduction: Why Targeted RA Treatments Matter
Rheumatoid arthritis (RA) is a chronic, autoimmune disease that affects approximately 1% of the global population. It causes persistent inflammation of the joints, resulting in pain, stiffness, swelling, and reduced mobility. Left untreated, RA can lead to permanent joint damage, disability, and decreased quality of life. Traditional disease-modifying antirheumatic drugs (DMARDs), such as methotrexate, have been the cornerstone of treatment for decades. However, many patients experience inadequate responses or intolerable side effects, necessitating newer, more precise therapies.
This growing need has driven the development of targeted biologics and small molecule inhibitors, particularly Janus kinase (JAK) inhibitors. JAK inhibitors work by interfering with specific signaling pathways responsible for immune activation and inflammation. Among these, Peficitinib Hydrobromide (development code: ASP015K) has emerged as a promising option, especially in patients with moderate-to-severe RA who have not responded adequately to conventional DMARDs.
Unlike broad immunosuppressants, targeted therapies like peficitinib selectively modulate immune activity, potentially reducing side effects while maintaining clinical efficacy. Approved in Japan and parts of Asia, peficitinib offers a convenient oral administration and has shown favorable safety and tolerability profiles in long-term clinical studies.
As treatment goals in RA evolve—shifting from symptom control to remission and prevention of joint destruction—novel therapies like peficitinib are positioned to play a crucial role. For patients and healthcare providers seeking alternatives to traditional therapies, understanding the benefits of targeted treatments is more important than ever.
What Is Peficitinib Hydrobromide?
Peficitinib Hydrobromide, also known by its development code ASP015K, is an orally administered small-molecule drug classified as a Janus kinase (JAK) inhibitor. It was developed to treat autoimmune diseases, with a primary focus on rheumatoid arthritis (RA)—particularly in patients who have not responded adequately to traditional disease-modifying antirheumatic drugs (DMARDs) like methotrexate.
Unlike conventional therapies, peficitinib selectively targets the JAK signaling pathway, which plays a key role in the body’s immune response. It inhibits multiple JAK isoforms—JAK1, JAK2, JAK3, and TYK2—disrupting the downstream signaling of pro-inflammatory cytokines such as interleukins and interferons. This selective mechanism helps reduce chronic inflammation while minimizing the broader immunosuppressive effects associated with older drugs.
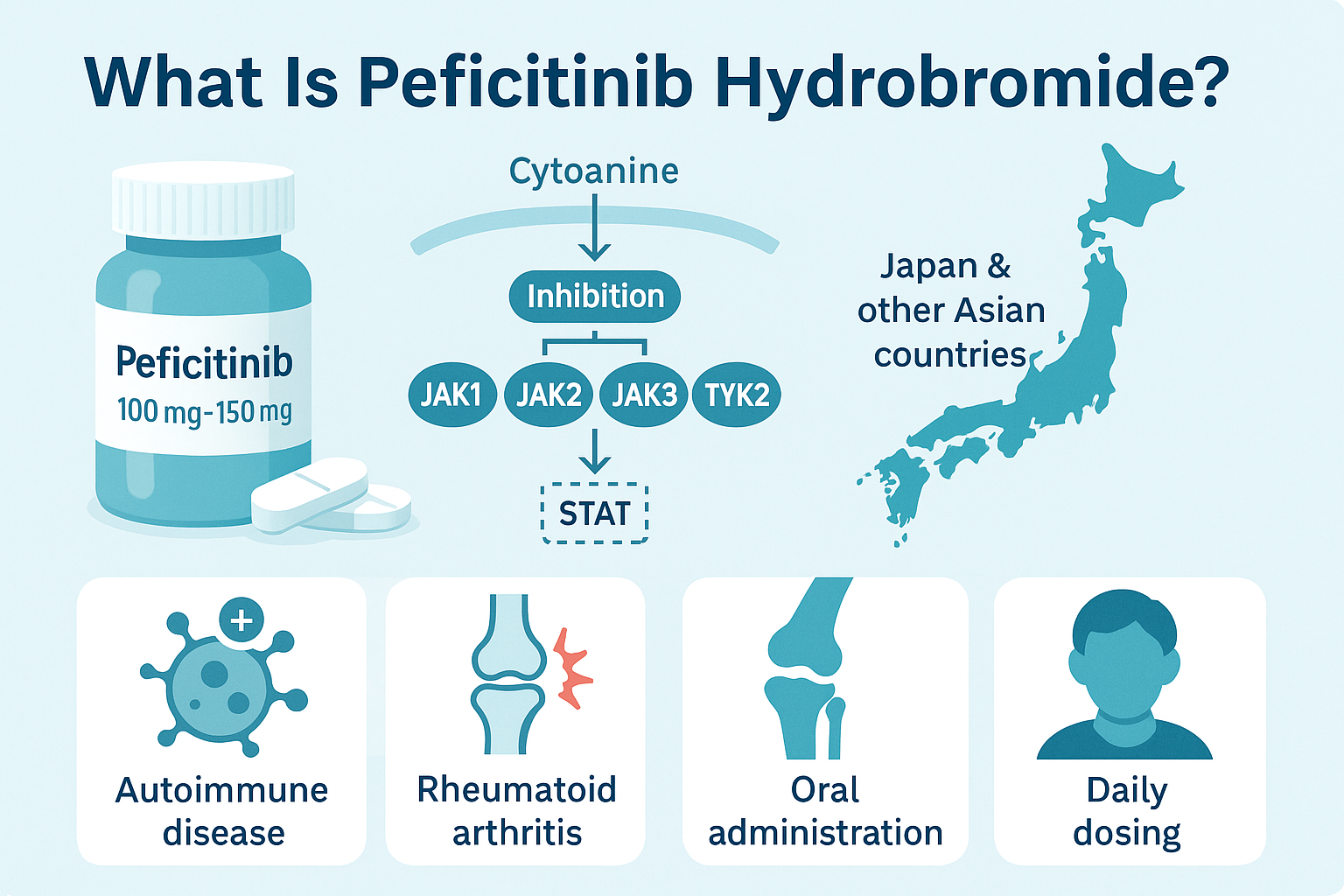
Peficitinib Hydrobromide is commercially available in Japan and some other Asian countries, where it has been approved for treating moderate-to-severe RA. It is commonly prescribed in 100 mg or 150 mg daily doses, with dose adjustments based on tolerance and disease severity. Its oral administration offers convenience over injectable biologics, making it a preferred option for some patients.
What makes peficitinib particularly interesting is its balanced efficacy and safety profile, especially in long-term use. Studies have shown that it is well-tolerated, even in elderly populations, with manageable side effects when monitored appropriately.
As of now, Peficitinib is not approved in the United States or European Union, but research continues regarding its potential in other autoimmune conditions and global markets. With growing interest in JAK inhibitors, peficitinib is seen as a promising option in the evolving landscape of targeted RA therapies.
How Peficitinib Works in the Body
Peficitinib Hydrobromide works by targeting a group of enzymes known as Janus kinases (JAKs), which are essential for transmitting signals from cytokine receptors on the cell surface to the nucleus. These signals regulate immune cell activity and inflammation—key drivers of rheumatoid arthritis (RA). By inhibiting JAK1, JAK2, JAK3, and TYK2, peficitinib disrupts the JAK-STAT signaling pathway, thereby reducing inflammatory responses at the cellular level.
When cytokines like interleukin-6 (IL-6) or interferon-gamma bind to their receptors, JAKs activate and phosphorylate STAT proteins, which then enter the nucleus and influence gene expression. This leads to the production of inflammatory proteins that cause joint damage in RA. Peficitinib interferes at this critical juncture, preventing overactivation of immune pathways without completely suppressing the immune system.
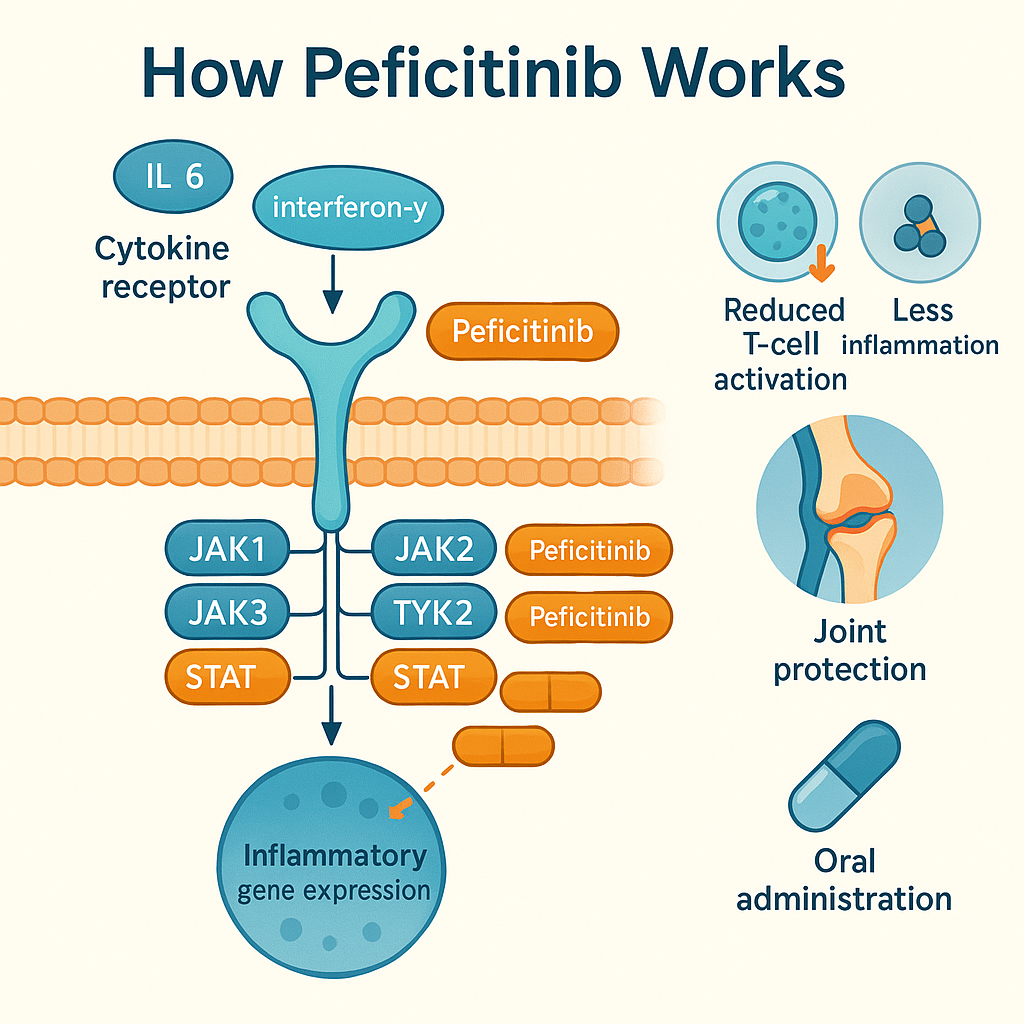
What sets peficitinib apart from other JAK inhibitors is its broad inhibitory profile across multiple JAK isoforms, offering a more comprehensive modulation of inflammation. While drugs like tofacitinib mainly target JAK1 and JAK3, peficitinib’s wider inhibition spectrum may contribute to its enhanced efficacy, especially in complex cases of RA.
In clinical models, peficitinib has demonstrated the ability to reduce T-cell proliferation, inhibit pro-inflammatory cytokines, and prevent joint swelling and erosion. Its mechanism is also associated with oral bioavailability and consistent plasma concentration, making it effective with once-daily dosing.
Understanding how peficitinib works helps explain both its therapeutic benefits and its potential side effects—such as increased infection risk due to immune suppression. This balance underscores the importance of appropriate patient selection and regular monitoring during treatment.
Clinical Benefits and Effectiveness of Peficitinib Hydrobromide
Peficitinib Hydrobromide has demonstrated strong clinical effectiveness in treating moderate to severe rheumatoid arthritis (RA), especially in patients with inadequate responses to methotrexate or conventional DMARDs. Across several Phase II and Phase III clinical trials, peficitinib has shown significant improvement in disease activity, joint function, and patient-reported outcomes.
One of the most cited studies is the RAJ3 trial, which evaluated the efficacy of peficitinib in Asian patients over 12 weeks. Patients receiving 100 mg or 150 mg of peficitinib daily showed statistically significant improvements in ACR20/50/70 response rates, compared to placebo. Notably, higher doses were associated with faster and more sustained reductions in Disease Activity Score-28 (DAS28) and C-reactive protein (CRP) levels.
Long-term extension studies (up to 2–3 years) have confirmed that peficitinib maintains consistent disease control, even in patients previously unresponsive to other therapies. In one pooled analysis involving over 1,000 patients, the median treatment exposure was more than 2 years, and remission or low disease activity was achieved and maintained in a significant percentage of cases.
Furthermore, patient- and physician-reported outcomes, including physical function and pain scores, also improved significantly. These improvements were consistent regardless of age, with elderly patients tolerating the drug well over time.
Peficitinib’s oral administration and stable pharmacokinetics make it especially appealing in long-term management strategies. It provides an alternative to injectable biologics, offering convenience and patient adherence benefits without sacrificing clinical efficacy.
Side Effects and Safety Considerations of Peficitinib Hydrobromide
While Peficitinib Hydrobromide has shown strong clinical effectiveness in treating rheumatoid arthritis (RA), it’s important to understand its side effects and safety profile, especially for long-term use.
The most commonly reported adverse events in clinical trials include upper respiratory tract infections, elevated liver enzymes, diarrhea, and headaches. These effects are generally mild to moderate in severity and can often be managed with dose adjustments or supportive care. Unlike some biologics, peficitinib is administered orally, which eliminates risks associated with injectable therapies but introduces the need for close systemic monitoring.
A key area of concern across all JAK inhibitors is the increased risk of infections, including herpes zoster (shingles). In pooled long-term studies, the incidence of herpes zoster was higher in peficitinib-treated patients, particularly those of Asian descent. Prophylactic vaccination and patient screening are often recommended before initiating therapy.
Peficitinib is also associated with elevations in lipid levels and creatine phosphokinase (CPK), though these changes are typically reversible and asymptomatic. Regular blood monitoring—particularly liver function tests and complete blood counts—is advised throughout treatment.
In comparison with other JAK inhibitors like tofacitinib and baricitinib, peficitinib has shown a comparable or slightly improved safety profile, especially at the 100 mg/day dose. Importantly, it appears to have less cardiovascular and thrombotic risk, although real-world data is still being collected.
Overall, peficitinib is considered safe and well-tolerated when used in appropriate patients and monitored according to guidelines. Physicians should evaluate the benefit-risk ratio, particularly in older adults or those with pre-existing comorbidities.
Conclusion
Peficitinib Hydrobromide represents a promising advancement in the treatment of rheumatoid arthritis, especially for patients who do not respond to conventional therapies like methotrexate. As a broad-spectrum JAK inhibitor, it effectively reduces inflammation by targeting multiple signaling pathways, offering both symptom relief and disease control. Its oral formulation adds convenience compared to injectable biologics, while long-term studies show encouraging safety and efficacy results, particularly in Asian populations. Although not yet approved worldwide, peficitinib is gaining recognition as a valuable option in personalized RA treatment strategies. As research continues, it may also prove useful in other autoimmune conditions. With careful patient monitoring, peficitinib offers a powerful, targeted alternative for managing this debilitating chronic disease.
References
Tanaka, Y., Izutsu, H. (2020). Peficitinib for the treatment of rheumatoid arthritis: An overview from clinical trials. Expert Opinion on Pharmacotherapy, 21(10), 1167–1176.
https://www.tandfonline.com/doi/full/10.1080/14656566.2020.1739649
Markham, A., & Keam, S. J. (2019). Peficitinib: First global approval. Drugs, 79(6), 699–704.
https://link.springer.com/article/10.1007/s40265-019-01131-y
Qiu, Q., Feng, Q., Tan, X., & Guo, M. (2019). JAK3-selective inhibitor peficitinib for the treatment of rheumatoid arthritis. Expert Review of Clinical Pharmacology, 12(7), 651–659.
https://www.tandfonline.com/doi/abs/10.1080/17512433.2019.1615443
Tanaka, Y., Okumura, H., Kim, S., & Dorey, J. (2021). Comparative efficacy of peficitinib vs. tofacitinib and baricitinib: A network meta-analysis. Rheumatology and Therapy, 8(2), 275–289.
https://link.springer.com/article/10.1007/s40744-021-00284-1




