Oveporexton (TAK-861): A Breakthrough Orexin Agonist for Narcolepsy Treatment
Abstract
Oveporexton (TAK-861) is a novel, oral orexin receptor 2 agonist developed by Takeda Pharmaceuticals for the treatment of Narcolepsy Type 1. Unlike traditional therapies, TAK-861 targets the core neurochemical cause of the disorder—orexin deficiency—offering a mechanism-based approach to restore natural wakefulness and reduce cataplexy. Clinical trials have shown significant improvements in daytime alertness and sleep stability with a favorable safety profile. As it moves toward FDA and EMA approval in 2026, TAK-861 is expected to transform narcolepsy treatment and expand into broader sleep disorder markets. Its unique profile, once-daily dosing, and strong efficacy data mark it as a future first-line therapy in sleep medicine.
What is Oveporexton (TAK-861)? A New Hope for Narcolepsy
Oveporexton (TAK-861) is a promising new drug candidate currently in late-stage clinical development for the treatment of Narcolepsy Type 1, a chronic neurological sleep disorder. Developed by Takeda Pharmaceuticals, TAK-861 represents a groundbreaking approach to treating narcolepsy by directly addressing the underlying cause of the disorder—orexin (hypocretin) deficiency.
Unlike traditional treatments, such as modafinil or sodium oxybate, which only manage symptoms like excessive daytime sleepiness or cataplexy, Oveporexton is the first selective Orexin Receptor 2 (OX2R) agonist designed to restore wakefulness by mimicking the action of the body’s natural orexin neuropeptides. Orexin plays a critical role in maintaining wakefulness and regulating the sleep–wake cycle. In individuals with Narcolepsy Type 1, these orexin-producing neurons are lost, leading to unstable transitions between sleep and wake states.
TAK-861 is an oral medication that can be taken once or twice daily, offering a more convenient and potentially more effective alternative to existing therapies. Recent clinical trial results published in the New England Journal of Medicine (2025) showed significant improvements in both wakefulness and reduction of cataplexy events among participants receiving Oveporexton.
As research continues, Oveporexton may not only redefine the treatment of narcolepsy but could also pave the way for new therapeutic strategies for other sleep disorders. Its mechanism-based design and robust clinical data make TAK-861 a major breakthrough in sleep medicine.
How TAK-861 Works: Mechanism of Action of an Orexin Receptor Agonist
TAK-861 (Oveporexton) is a selective agonist of the Orexin Receptor 2 (OX2R), making it a first-in-class therapy that addresses the root cause of Narcolepsy Type 1: a loss of orexin-producing neurons in the hypothalamus. Orexin (also known as hypocretin) is a neuropeptide responsible for promoting wakefulness, stabilizing sleep-wake transitions, and regulating energy balance.

In people with Narcolepsy Type 1, the brain produces little or no orexin, leading to excessive daytime sleepiness, sudden loss of muscle tone (cataplexy), and disrupted nighttime sleep. Unlike traditional stimulants that work on downstream neurotransmitters like dopamine or norepinephrine, TAK-861 directly activates OX2R, restoring natural wakefulness patterns by mimicking orexin’s effect.
The selectivity for OX2R is critical. While two receptors—OX1R and OX2R—exist, OX2R is more involved in wakefulness and vigilance. By targeting only OX2R, TAK-861 aims to minimize off-target effects like anxiety or cardiovascular stimulation, commonly seen with other stimulants.
Moreover, TAK-861 is administered orally, offering ease of use and improved patient compliance compared to intravenous agents like danavorexton. Preclinical studies showed strong blood-brain barrier penetration, and human trials have demonstrated sustained receptor engagement and functional improvements in narcolepsy symptoms.
TAK-861’s mechanism offers not just symptom control, but mechanism-based therapy that may redefine treatment for sleep-wake disorders.
Clinical Trial Results: How Effective Is Oveporexton (TAK-861)?
TAK-861 (Oveporexton) has shown remarkable clinical efficacy in the treatment of Narcolepsy Type 1, as demonstrated by recent results from a Phase III randomized, double-blind, placebo-controlled trial, published in the New England Journal of Medicine in 2025.
The study enrolled patients diagnosed with Narcolepsy Type 1, all of whom experienced chronic excessive daytime sleepiness (EDS) and cataplexy—the hallmark symptoms of the condition. Participants received either once- or twice-daily doses of TAK-861 or a placebo over a multi-week treatment period.
Key Findings:
Significant reduction in EDS, as measured by the Maintenance of Wakefulness Test (MWT) and Epworth Sleepiness Scale (ESS)
Marked decrease in cataplexy attacks per week compared to baseline
Rapid onset of action, with many patients experiencing improvement within the first week
Favorable safety profile, with the most common side effects being mild headache and transient nausea
What makes these results so promising is that Oveporexton not only manages symptoms but also targets the underlying neurochemical deficiency in orexin signaling. This makes TAK-861 a mechanism-based treatment rather than a symptomatic band-aid.
Unlike current therapies like modafinil, pitolisant, or sodium oxybate—which work by influencing secondary neurotransmitter systems—TAK-861 works upstream at the orexin receptor level, offering more natural, sustained wakefulness with reduced rebound effects or sleep disruption.
Given the strength of these findings, TAK-861 is being hailed as a potential first-line therapy once approved by regulatory authorities.
Why TAK-861 Matters: Redefining Narcolepsy Treatment
The development of TAK-861 (Oveporexton) marks a major shift in the treatment landscape for Narcolepsy Type 1. Unlike existing therapies that merely mask symptoms, TAK-861 is the first oral medication to target the root cause of the disorder: orexin deficiency.
For decades, narcolepsy has been managed with symptomatic treatments such as modafinil (a stimulant), sodium oxybate (a sedative), and antidepressants for cataplexy. While helpful, these drugs often require multiple daily doses, have abuse potential, or cause significant side effects like disturbed sleep or mood changes. They also do not restore the brain’s normal sleep-wake architecture.
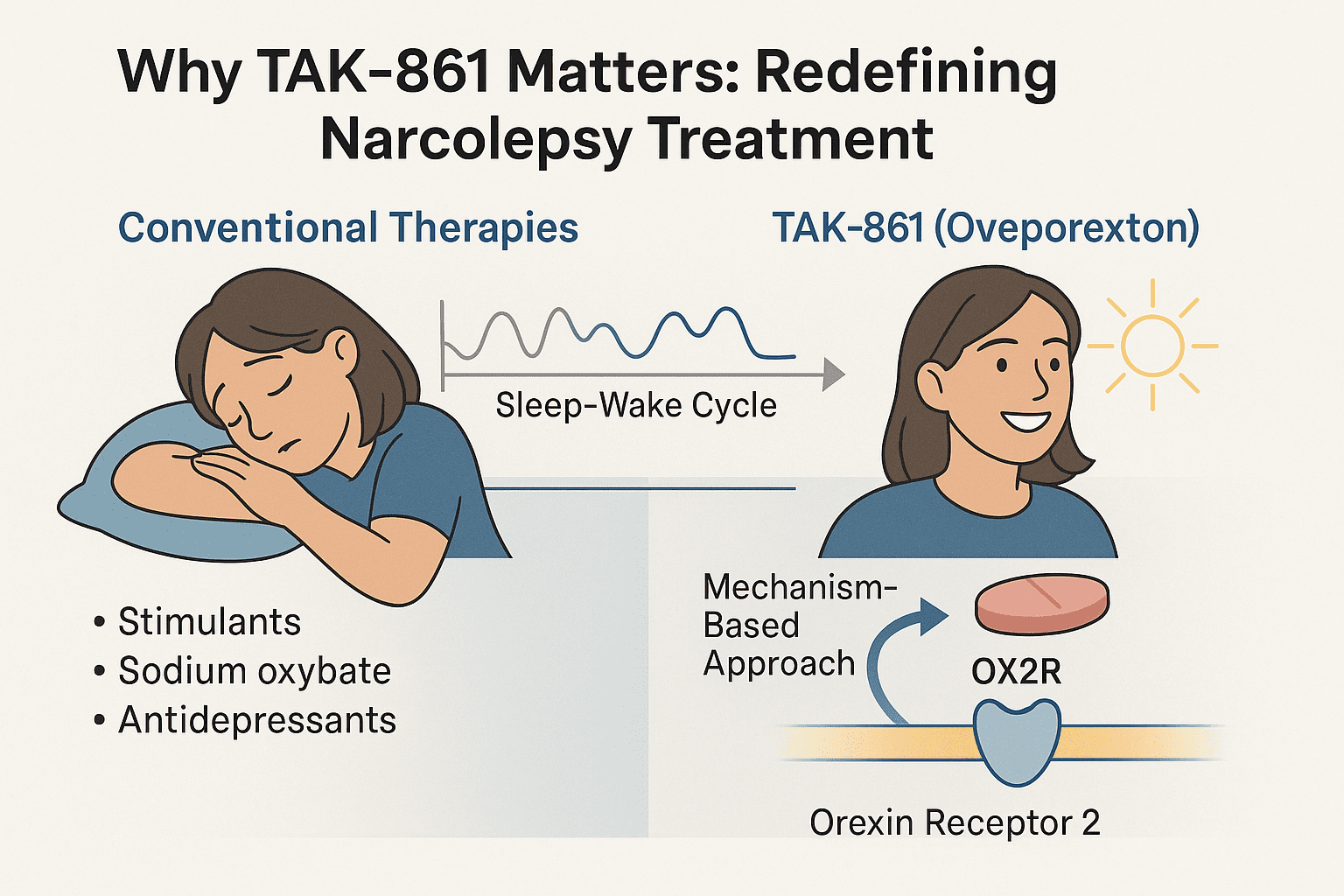
TAK-861 offers a mechanism-based solution. By selectively activating the OX2R receptor, it simulates the function of natural orexin signaling, stabilizing the sleep–wake cycle from its source. This direct mechanism could potentially offer more consistent wakefulness, fewer cataplexy episodes, and better nighttime sleep—without the roller-coaster effect often experienced with current medications.
What sets TAK-861 apart is also its oral route of administration, eliminating the need for intravenous infusions like danavorexton. Its potential for once-daily dosing improves patient compliance and daily quality of life.
In essence, Oveporexton not only represents a pharmacological innovation, but also restores biological balance in a condition long managed through workaround strategies. With its strong trial results and targeted action, TAK-861 is positioned to become the first-line treatment for Narcolepsy Type 1—and possibly, in the future, other sleep-wake disorders.
What’s Next for TAK-861: Approval Timeline and Market Outlook
With the successful completion of Phase III clinical trials, TAK-861 (Oveporexton) is now on the fast track toward regulatory approval. Based on strong efficacy data and a favorable safety profile, Takeda Pharmaceuticals has confirmed that it intends to submit New Drug Applications (NDAs) to the FDA and EMA by late 2025, aiming for market launch in 2026.
This positions Oveporexton to become the first oral orexin receptor 2 agonist available for Narcolepsy Type 1, and potentially the first disease-modifying treatment in the space.
Market Impact and Commercial Potential
The global narcolepsy treatment market is projected to exceed $4.5 billion USD by 2030, driven by increasing diagnosis rates, improved awareness, and demand for better-tolerated, once-daily medications. Oveporexton’s unique mechanism and convenient oral formulation make it a highly competitive alternative to existing therapies like modafinil, pitolisant, and sodium oxybate.
Analysts expect TAK-861 to:
Rapidly gain market share as a first-line treatment for NT1
Expand to related indications such as Narcolepsy Type 2 and Idiopathic Hypersomnia
Potentially be used in off-label cases involving excessive daytime sleepiness (EDS) in other neurological conditions
Takeda is also expected to launch educational and market access initiatives to support adoption and payer coverage, further reinforcing its positioning in the sleep disorder therapeutics market.
With its science-driven approach, patient-friendly profile, and market readiness, TAK-861 is poised to transform how narcolepsy is treated—both clinically and commercially.
References
Dauvilliers, Y., Plazzi, G., Mignot, E., et al. (2025). Oveporexton, an Oral Orexin Receptor 2–Selective Agonist, in Narcolepsy Type 1. New England Journal of Medicine.
https://www.nejm.org/doi/abs/10.1056/NEJMoa2405847
Mullard, A. (2025). Leading orexin receptor agonist clears phase III for narcolepsy. Nature Reviews Drug Discovery.



