Nintedanib (Ofev): Everything You Need to Know About This Anti-Fibrotic Agent
Abstract
Nintedanib esylate is a multi-targeted tyrosine kinase inhibitor used to treat fibrotic and cancer-related diseases, most notably idiopathic pulmonary fibrosis (IPF), systemic sclerosis-associated ILD, and advanced non-small cell lung cancer (NSCLC). It works by inhibiting key growth factor receptors—VEGFR, FGFR, and PDGFR—thereby reducing fibrosis and angiogenesis. Administered orally in capsule form, the drug is generally well-tolerated with manageable side effects such as diarrhea and elevated liver enzymes. Recent research is expanding its therapeutic potential into liver fibrosis, oncology, and biomarker-driven treatments. As a cornerstone in anti-fibrotic therapy, Nintedanib continues to be the subject of intense clinical investigation and development.
What is Nintedanib Esylate?
Nintedanib esylate is a targeted therapeutic agent used primarily to treat fibrotic and cancerous diseases. It is the ethanesulfonate salt form of nintedanib, a multi-kinase inhibitor that blocks several growth factor receptors involved in the proliferation and migration of fibroblasts and tumor cells. This drug is commercially known under the brand name Ofev®, manufactured by Boehringer Ingelheim, and has gained approval in multiple countries for the treatment of idiopathic pulmonary fibrosis (IPF), systemic sclerosis-associated interstitial lung disease (SSc-ILD), and other chronic fibrosing interstitial lung diseases.
Chemically, Nintedanib is a small molecule inhibitor targeting VEGFR (vascular endothelial growth factor receptors 1–3), PDGFR (platelet-derived growth factor receptors α and β), and FGFR (fibroblast growth factor receptors 1–3). This makes it effective not only in fibrotic lung conditions but also as a second-line treatment in non-small cell lung cancer (NSCLC), where it is used in combination with chemotherapy agents like docetaxel.
The esylate form of Nintedanib offers improved solubility and bioavailability, which contributes to its efficacy and ease of formulation as an oral soft capsule. Since its approval by the FDA in 2014 for IPF, the drug has been at the center of ongoing research and development due to its dual anti-fibrotic and anti-angiogenic properties.
As a result, Nintedanib esylate represents a significant advancement in the management of chronic fibrotic diseases, where treatment options have historically been limited and largely palliative.
How Does Nintedanib Esylate Work?
Nintedanib esylate works by targeting and inhibiting receptor tyrosine kinases (RTKs) that are critically involved in fibrosis and angiogenesis, two pathological processes central to diseases like idiopathic pulmonary fibrosis (IPF) and certain cancers. Specifically, Nintedanib blocks signaling from vascular endothelial growth factor receptors (VEGFR 1–3), platelet-derived growth factor receptors (PDGFR α and β), and fibroblast growth factor receptors (FGFR 1–3).
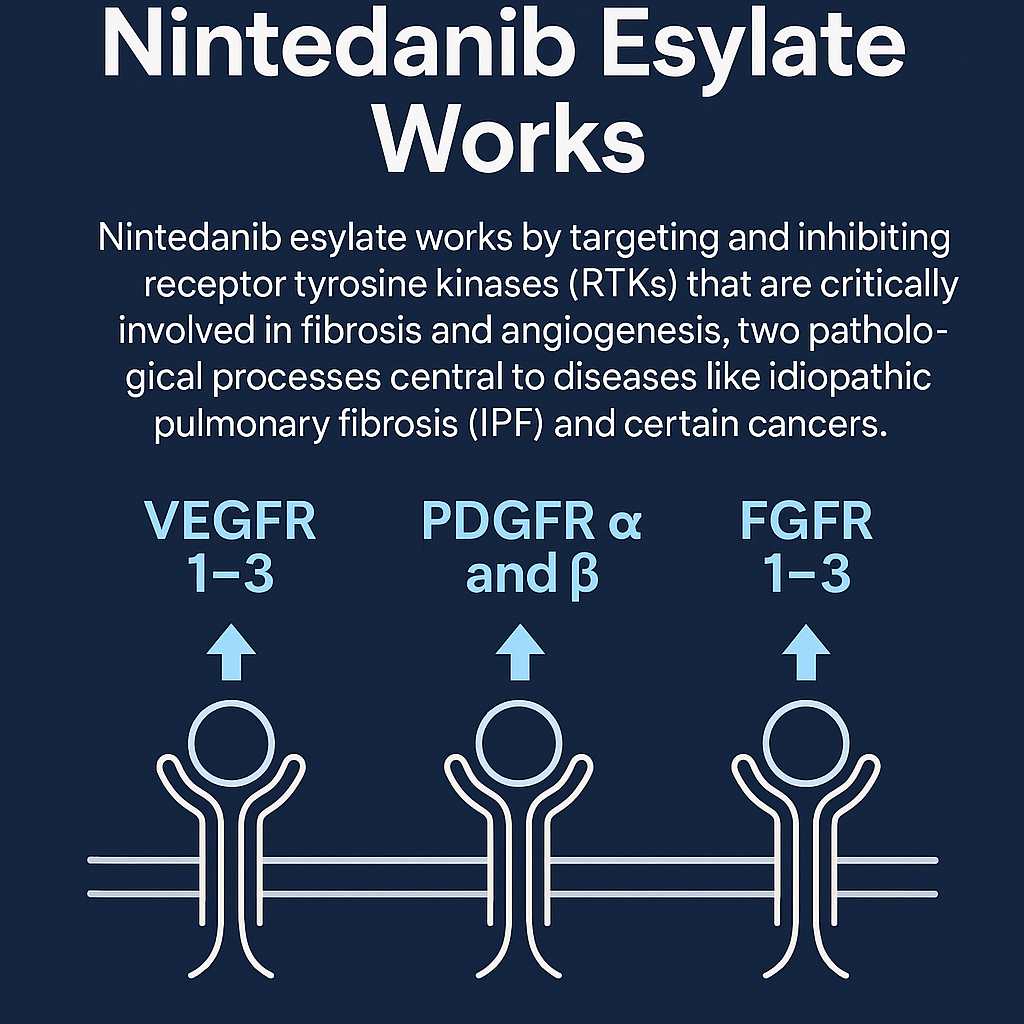
These growth factor pathways are normally involved in the regulation of cell proliferation, differentiation, angiogenesis, and tissue repair. However, in chronic fibrosing diseases, they become dysregulated, leading to excessive fibroblast activity, extracellular matrix deposition, and scarring. By inhibiting these kinases, Nintedanib reduces fibroblast migration and proliferation, limits angiogenesis, and slows disease progression.
In oncology, particularly non-small cell lung cancer (NSCLC), the inhibition of angiogenesis impedes tumor vascularization, thereby starving cancer cells of oxygen and nutrients. When used with cytotoxic agents like docetaxel, Nintedanib enhances antitumor efficacy by modifying the tumor microenvironment.
Nintedanib is classified as a triple angiokinase inhibitor, and its multi-targeted approach distinguishes it from single-pathway agents. Importantly, it exhibits sustained target inhibition, contributing to long-term disease modulation. Due to its oral bioavailability and manageable safety profile, it is widely accepted as a front-line or adjunct therapy in fibrotic and neoplastic conditions.
Approved Uses and Therapeutic Indications
Nintedanib esylate has emerged as a cornerstone treatment for several fibrotic and malignant diseases, with approvals backed by robust clinical evidence. Its first and most recognized use is in the treatment of idiopathic pulmonary fibrosis (IPF), a chronic, progressive lung disease characterized by irreversible scarring of lung tissue. Nintedanib slows the decline in lung function by inhibiting fibrogenic pathways and is one of only two drugs approved for this condition globally.
The drug is also approved for systemic sclerosis-associated interstitial lung disease (SSc-ILD), where it targets fibrotic activity in patients with autoimmune-mediated lung damage. In clinical trials, it has significantly reduced the rate of forced vital capacity (FVC) decline, a key measure of lung function.
In 2020, the FDA approved Nintedanib for chronic fibrosing interstitial lung diseases with a progressive phenotype, expanding its use to a broader patient population suffering from a variety of rare but progressive lung conditions.
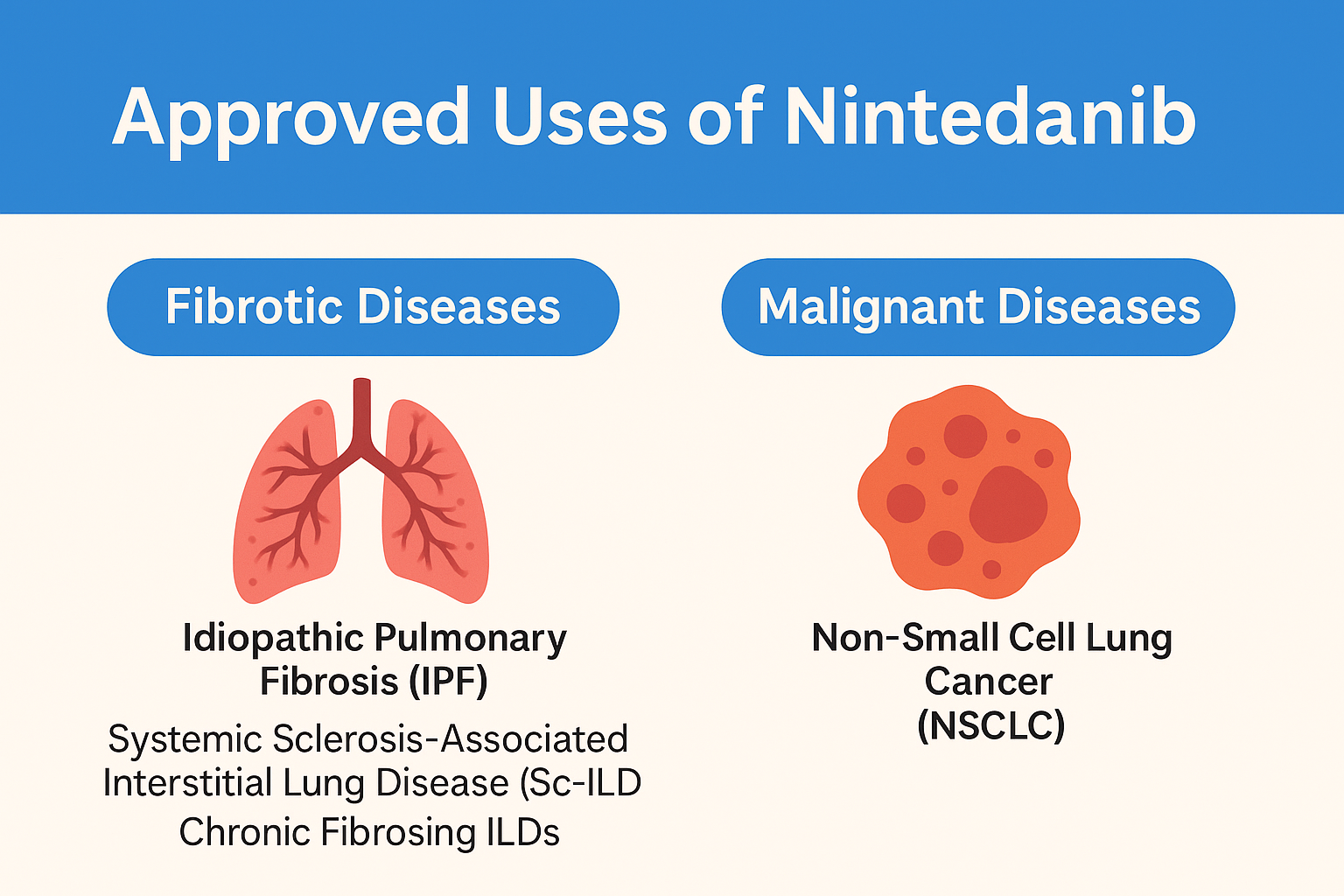
Additionally, Nintedanib has an oncological indication for non-small cell lung cancer (NSCLC). Specifically, it is approved in the European Union for use in combination with docetaxel for advanced adenocarcinoma after failure of first-line chemotherapy. Its anti-angiogenic effect helps to curb tumor progression and metastasis.
These approvals underline Nintedanib’s role as both an anti-fibrotic and anti-cancer agent, with its multi-kinase inhibition mechanism offering a broad-spectrum therapeutic strategy.
Potential Side Effects and Safety Profile
Like most potent kinase inhibitors, Nintedanib esylate is associated with a range of side effects, which vary in intensity from mild to severe. The most commonly reported adverse event is diarrhea, occurring in up to 60% of patients. Other gastrointestinal symptoms include nausea, vomiting, and abdominal pain. These side effects are often manageable with dose adjustments or supportive therapy.
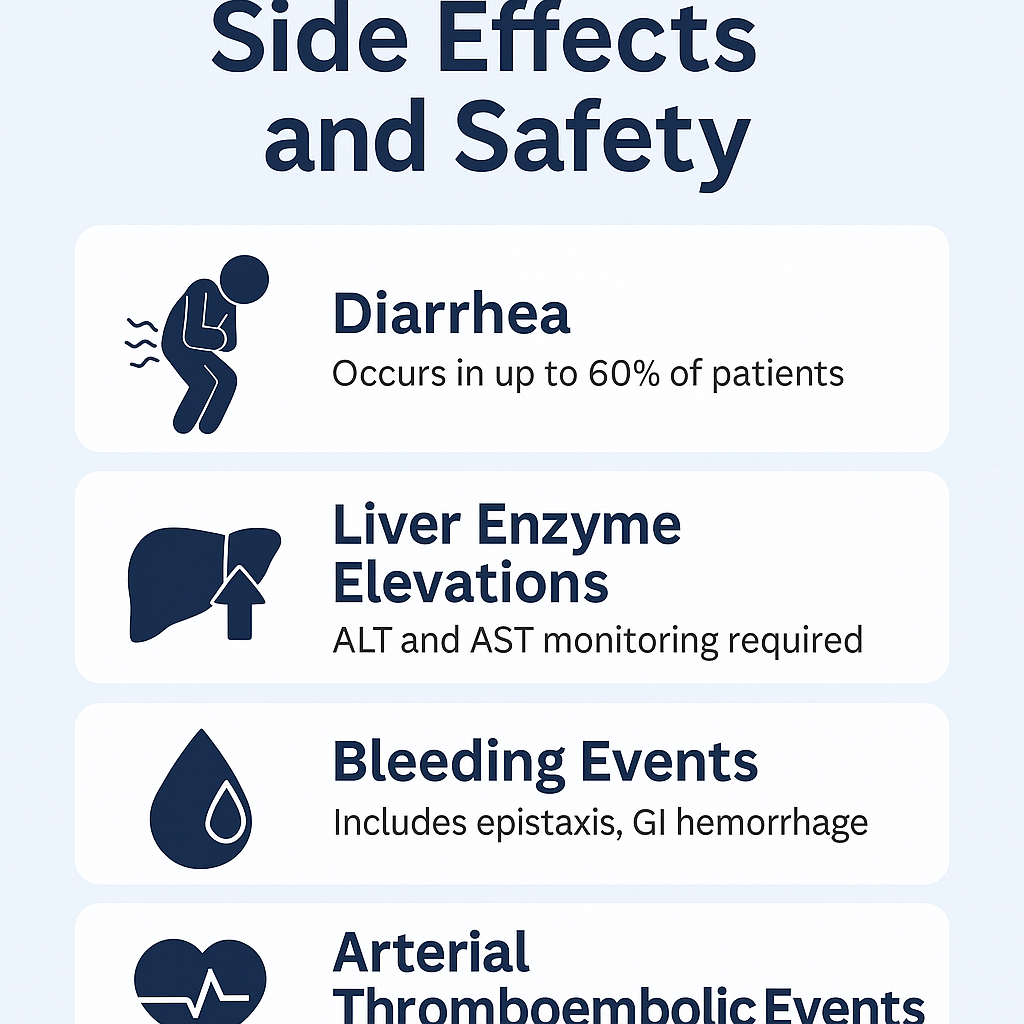
Another important concern is elevated liver enzymes, particularly ALT and AST. Regular monitoring of liver function is recommended before treatment initiation and periodically during therapy. In some cases, treatment interruption or dose reduction may be required.
Bleeding events, including epistaxis and gastrointestinal hemorrhage, have been observed, likely due to the anti-angiogenic mechanism of action. Therefore, caution is advised in patients on anticoagulation therapy or with known bleeding risks.
Less frequently, arterial thromboembolic events, such as myocardial infarction, have been reported. Patients with underlying cardiovascular disease should be closely monitored.
Although rare, interstitial lung disease (ILD) has been associated with nintedanib treatment in cancer patients, and distinguishing between disease progression and drug-related effects is critical.
Despite these risks, nintedanib is generally well tolerated with appropriate patient selection and monitoring. Education on recognizing early symptoms and timely dose modification plays a vital role in maintaining safety and treatment adherence.
Future Perspectives and Research Developments
Nintedanib esylate is under active investigation for a broad range of fibrotic and oncologic conditions, beyond its currently approved indications. One of the most promising areas is its use in liver fibrosis, particularly non-alcoholic steatohepatitis (NASH), where preclinical studies have shown favorable modulation of fibrogenic pathways.
In oncology, Nintedanib is being evaluated in combination with immunotherapy and chemotherapeutic agents, targeting resistant or metastatic tumors such as ovarian, colorectal, and pancreatic cancers. Its anti-angiogenic properties make it a compelling agent in tumor environments where vascular remodeling is a key survival mechanism.
Another area of interest is in combination regimens with other anti-fibrotic agents, such as pirfenidone, to explore synergistic effects in treating complex fibrosing interstitial lung diseases.
Clinical trials are also examining biomarker-driven patient selection, aiming to optimize outcomes by identifying populations that are more likely to respond to multi-kinase inhibition. Personalized medicine approaches, incorporating gene expression and molecular profiling, are on the horizon.
Furthermore, efforts are being made to develop alternative formulations, including inhaled versions, which may reduce systemic side effects and deliver more targeted lung therapy.
These developments position Nintedanib as a versatile platform drug with expanding utility across medicine and offer hope in areas of unmet clinical need.
References
Richeldi, L., du Bois, R. M., Raghu, G., Azuma, A., Brown, K. K., Costabel, U., … & Collard, H. R. (2014). Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. New England Journal of Medicine, 370(22), 2071-2082.
https://www.nejm.org/doi/full/10.1056/NEJMoa1402584
Wollin, L., Wex, E., Pautsch, A., Schnapp, G., Hostettler, K. E., Stowasser, S., & Kolb, M. (2015). Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. European Respiratory Journal, 45(5), 1434-1445. https://doi.org/10.1183/09031936.00174914
https://publications.ersnet.org/content/erj/45/5/1434.abstract
Distler, O., Highland, K. B., Gahlemann, M., Azuma, A., Fischer, A., Mayes, M. D., … & Tashkin, D. P. (2019). Nintedanib for systemic sclerosis-associated interstitial lung disease. New England Journal of Medicine, 380(26), 2518-2528. https://doi.org/10.1056/NEJMoa1903076
https://www.nejm.org/doi/full/10.1056/NEJMoa1903076
Hilberg, F., Tontsch-Grunt, U., Baum, A., Le, A. T., & Doebele, R. C. (2008). BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Research, 68(12), 4774-4782.
https://doi.org/10.1158/0008-5472.CAN-07-6307




