News of Hydantoin-Based Universal Peptidomimetics
Abstract
Currently, Alessio’s team published a paper “Synthesis and Conformational Analysis of Hydantoin-Based Universal Peptidomimetics” to discuss the use, synthesis, and confirmation of Hydantoin-Based structures.[1] In 1988, Nobel Laureate Sir James Whyte Black asserted that the most effective approach to discovering new drugs is by building upon existing ones.
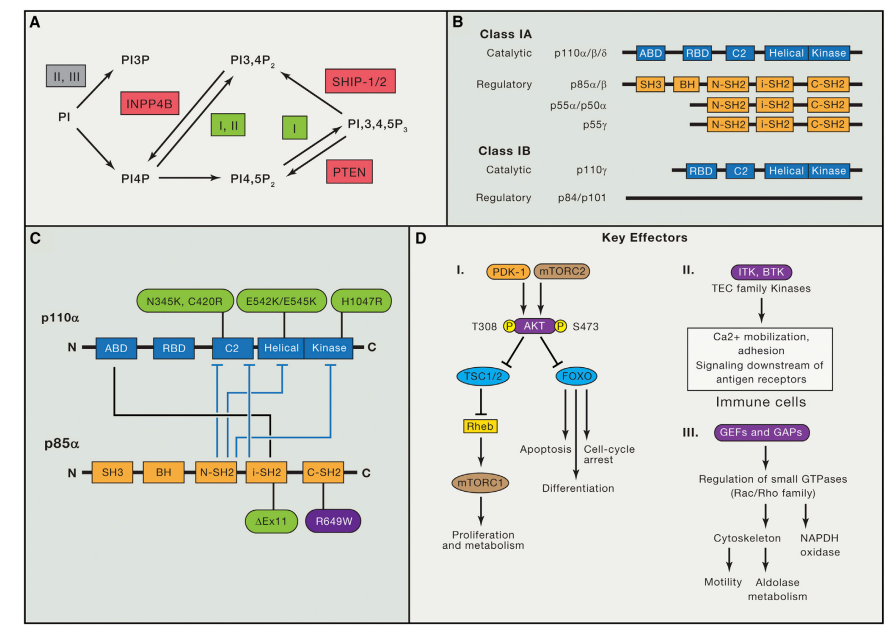
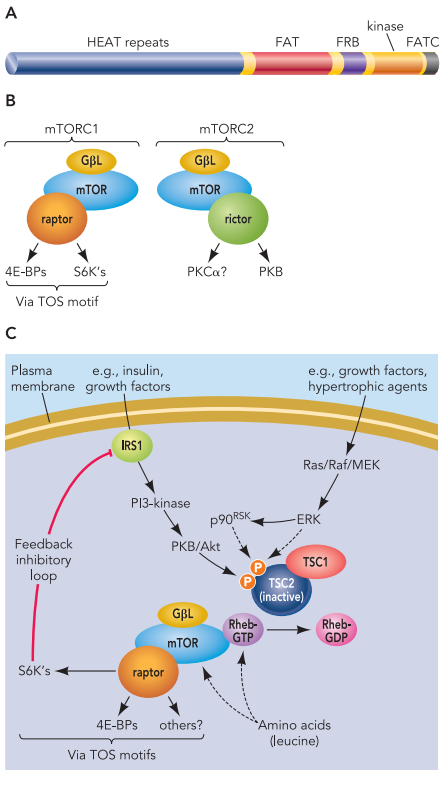
Even after almost 35 years, this statement remains pertinent. The concept of privileged scaffolds, notably hydantoin-based compounds, has emerged as a valuable strategy in medicinal chemistry. These scaffolds, prevalent in various synthetic drugs, offer templates for generating diverse bioactive molecules that can target multiple receptor types, even traditionally challenging “undruggable” targets.[2] Through meticulous decoration, primarily involving heterocycles, these scaffolds have proven instrumental in drug development, evolving from early concepts like “lock and key” theory and combinatorial chemistry to modern approaches like drug repositioning, privileged substructure-based diversity-oriented synthesis (pDOS), biology-oriented synthesis (BIOS), and the complexity-to-diversity (CtD) strategy.[3] Such privileged scaffolds act as “chemical navigators,” facilitating the design of targeted libraries and exploring uncharted chemical territory.
Furthermore, privileged scaffolds have been harnessed to design structural peptidomimetics, including minimalist and universal mimetics. These peptidomimetics, distinct from peptides, utilize a privileged scaffold lacking peptide characteristics as a foundation to construct structures mimicking protein secondary structures like α-helix and β-turn.[4] Notable examples include pyrrolidine, pyridazine, pyrrolopyrimidines, and others. These minimalist mimetics assume conformations with energies akin to global minima, aligning their substituents with key i + n positions of α-helices or β-turns. More recently, the concept of universal peptidomimetics has arisen, involving structurally adaptable scaffolds capable of mimicking various secondary structures by rotating around pivotal degrees of freedom[5]. These universal peptidomimetics, readily functionalized with side chain-like substituents, hold promise for designing libraries for high-throughput screening programs, even against traditionally challenging “undruggable” targets.
Among these scaffolds, hydantoin stands out. It is a small heterocycle possessing four derivatizable positions, enabling diversity and accommodating up to four hydrogen-bond donors/acceptors. Hydantoin-based compounds constitute a fascinating class of privileged scaffolds, displaying a wide range of biological activities. These compounds have been successfully employed as anticonvulsants, muscle relaxants, and agonists for androgen receptors, along with clinical candidates for treating psoriasis, LFA-1 antagonism, and Duchenne muscular dystrophy. Despite its classification as an “old” privileged scaffold, hydantoin’s synthetic and pharmaceutical interest has remained robust over the years. Various publications and patents have emerged, exploring both methodological and medicinal chemistry aspects. Notably, the hydantoin core serves as a privileged skeleton for designing minimalist and universal peptidomimetics.
To exemplify this, the text delves into the design and conformational analysis of racemic universal peptidomimetic 3-cyclo-butylcarbamoyl hydantoins. These compounds are synthesized through a regioselective multicomponent domino process, originating from α-azido-cyclo-butyl carboxylic ester and isocyanates. Modeling experiments reveal that these compounds can adopt α-helix and β-turn conformations at room temperature, enabling them to interchange without substantial entropic penalties. Their substituents (R1, R2, and R3) align with the key i + n side-chain positions of secondary structures.[6] To enhance the understanding of the stabilization role played by the exo-quaternary carbon and broaden the synthetic potential of universal peptidomimetics, the text presents the synthesis and conformational analysis of analogues with varied quaternary substituents (gem-dimethyl, cyclo-hexyl, and cyclo-propyl) and those lacking the substituent (glycine derivatives). A chemo-selective process involving liquid-liquid acid/base extractions is employed for isolation and purification, offering a suitable approach for combinatorial synthesis. This method yields enantiomerically pure derivatives, a departure from racemic mixtures produced by the previously utilized multicomponent process.
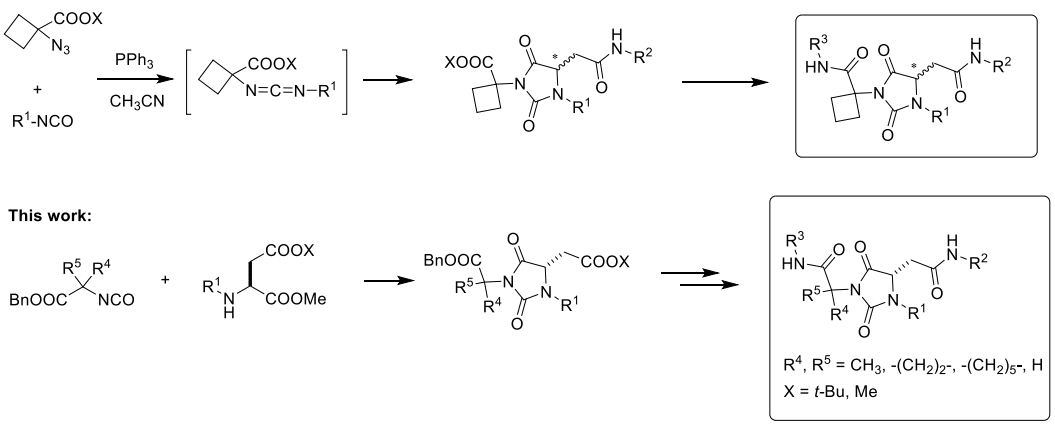
Scheme 1. Synthetic Strategies for the Synthesis of Hydantoin-Based Universal Peptidomimetics[6]
In summary, the text emphasizes the enduring significance of building upon established drugs and underscores the role of privileged scaffolds, particularly hydantoin-based compounds, in modern drug discovery. The ability of these scaffolds to mimic protein secondary structures holds great promise for the development of diverse and effective bioactive molecules.
Synthesis method of Hydantoin-Based chemicals
This paper discusses the synthesis and regioselective cyclization of hydantoin-based universal peptidomimetics through a multistep process. The initial approach, known as the MC process, utilizes an aza-Michael addition reaction to form O-acyl isourea intermediates, which then undergo regioselective cyclization. This cyclization is influenced by the steric hindrance of amino moieties, resulting in structurally diverse hydantoin scaffolds. The text also explores variations of the process using different azide substituents, aiming to achieve regiocontrol and high yields.
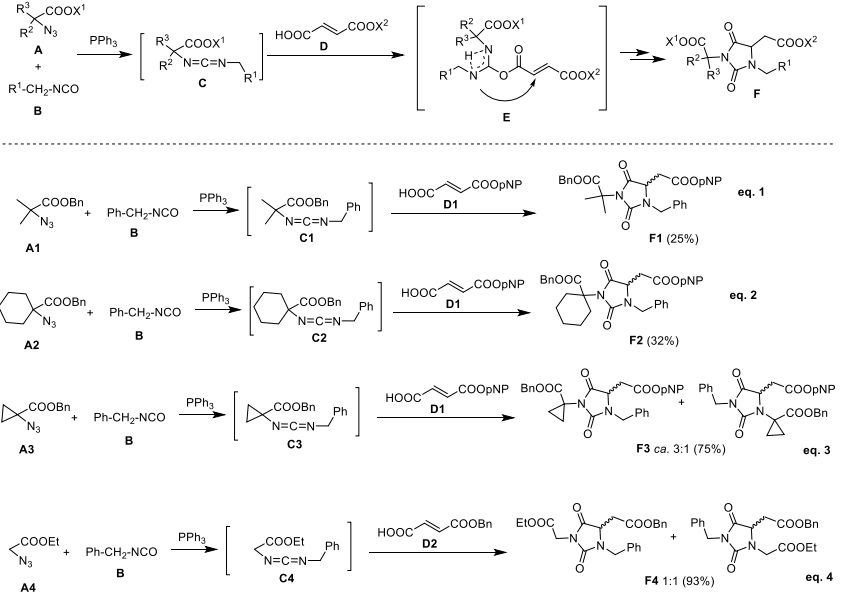
Scheme 2. MC Process Leading to Hydantoin F
However, due to limitations in regioselectivity and yields with certain azide derivatives, an alternative synthetic route is proposed. This alternative route involves the reaction between isocyanates and N-alkyl-α-amino esters, leading to a condensation/cyclization domino process.[7] The synthesis of N,N′-disubstituted hydantoins through this method is demonstrated, showing successful regioselective cyclization and high yields. The process is applied to generate a collection of systematically modified hydantoin derivatives, allowing for diverse substitution patterns and carboxyl group protection. Notably, the stereochemical instability of enantiomerically pure α-aminoester isocyanates is highlighted, leading to diastereoisomeric products.
Overall, the text emphasizes the importance of regioselective cyclization in the synthesis of hydantoin-based peptidomimetics and presents an alternative approach for generating structurally diverse compounds suitable for further functionalization and potential use in drug development.
Summary
In summary, scientists have developed a novel scaffold for universal peptidomimetics based on the hydantoin ring. The synthetic approach involves a chemoselective domino condensation/cyclization process between α-aminoester isocyanates and N-alkyl aspartic acid diesters, taking place under mild conditions (room temperature), followed by conventional deprotection/coupling reactions. This method allows for the recovery of intermediates through efficient liquid-liquid acid/base extraction, eliminating the need for further chromatographic purification.
Scientists successfully synthesized a diverse collection of 18 enantiomerically pure hydantoins with systematic substitutions, including variations in the quaternary carbon at the exo-C3 position (cyclo-hexyl, cyclo-propyl, gem-dimethyl) or a more flexible methylene group. The synthetic strategy accommodates a wide range of natural or unnatural amino acid side chains, derived from readily available aldehydes (R1) and amines (R2 and R3). This makes the approach particularly suitable for combinatorial synthesis and high-throughput screening programs.
Conformational studies were conducted using molecular modeling, solution-state NMR, CD, IR experiments, and solid-state X-ray analysis. Molecular modeling revealed that the scaffolds can adopt intramolecular hydrogen-bond-driven conformations resembling key side chains of protein secondary structures, such as α-helix and β-turn. Notably, more flexible glycine derivatives favored the α-helix conformation, while experiments on molecules with two hydrogens bonded to the C-3 carbon showed a stronger intramolecular hydrogen bond associated with both α-helix and β-turn conformations. X-ray analysis confirmed the adoption of a well-defined β-turn conformation in the solid state.
Collectively, these findings demonstrate that hydantoin scaffolds represent a novel class of universal peptidomimetics capable of adopting common protein secondary structures with favorable thermodynamic profiles.