New Hope for Hot Flashes: Elinzanetant Offers Relief Without Hormones
Abstract
Elinzanetant is a first-in-class, non-hormonal dual neurokinin (NK-1/NK-3) receptor antagonist developed to treat vasomotor symptoms (VMS) like hot flashes in menopausal women. It targets overactive KNDy neurons in the hypothalamus, restoring temperature regulation disrupted by declining estrogen. Clinical trials (OASIS-1, -2, -3) demonstrated significant, sustained reduction in VMS severity and frequency with a favorable safety profile. The drug addresses a major unmet need, as 65% of women remain untreated and many cannot take hormone therapy. With 1.2 billion women expected to enter menopause by 2030, elinzanetant offers a breakthrough therapy with global potential and marks a new era in women’s health treatment.
VMS clinical needs urgently need to be met
Menopausal vasomotor symptoms, namely hot flashes, are the most common symptoms of menopause, and traditional treatments face many difficulties. The use of hormone therapy is easily restricted. Since long-term use increases the risk of thrombosis, it is prohibited for breast cancer patients. However, non-hormonal drugs are scarce, and existing drugs are not effective enough. Antidepressants are effective in relieving hot flashes at about 40-60%.
Currently, about 65% of patients do not receive treatment, and about 20% of patients have symptoms that last for more than 10 years. Hot flashes at night cause insomnia in 80% of patients, and the risk of depression increases by 2 times. There is an urgent clinical need to be met.
Professor Paula Briggs, former president of the British Menopause Society, pointed out: “Hot flashes are not only a physical discomfort, but also destroy women’s work ability and mental health.”
Elinzanetant mechanism of action
Elinzanetant is a first-in-class dual neurokinin (NK) targeted therapy, specifically an NK-1 and NK-3 receptor antagonist.
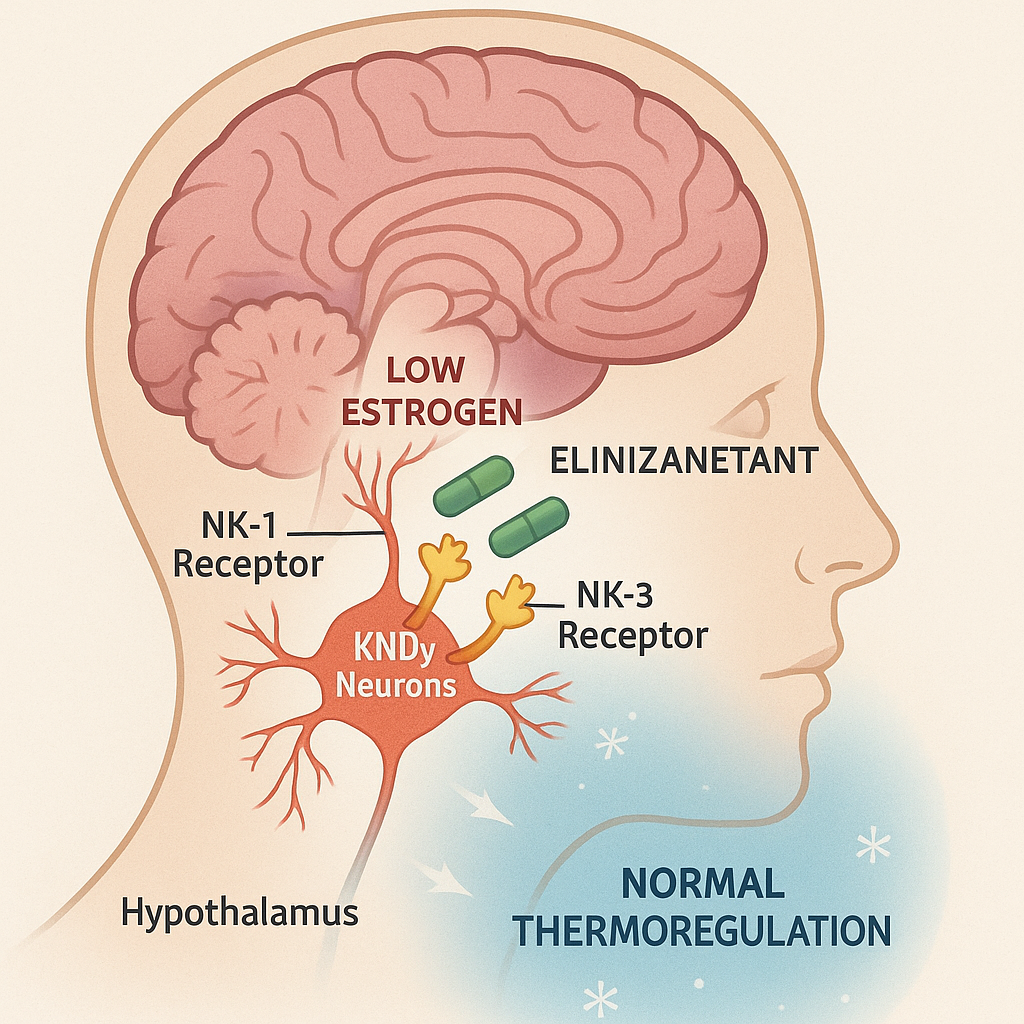
The drug treats VMS by regulating a group of estrogen-sensitive neurons (KNDy neurons) in the hypothalamus region of the brain. These neurons become hypertrophic as estrogen decreases, leading to overactivation of the thermoregulatory pathway, which disrupts the thermoregulatory mechanism and causes VMS. Elinzanetant relieves VMS by blocking NK-1 and NK-3 receptors and reducing overactivation of KNDy neurons.
Drug clinical trial data
The approval of elinzanetant is based on its positive efficacy and good safety in the Phase 3 clinical studies OASIS-1, -2 and -3.
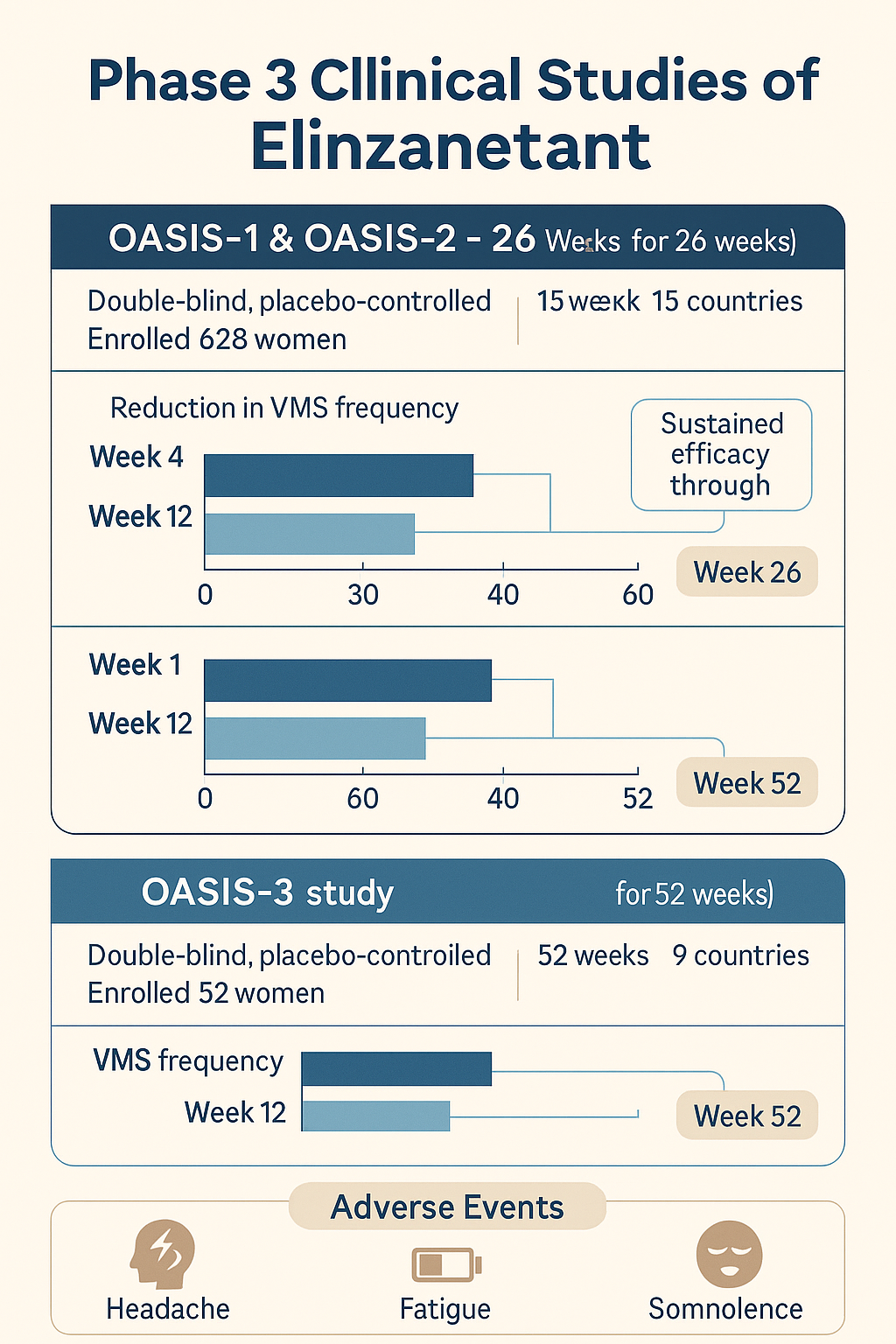
OASIS 1 and 2 are double-blind, randomized, placebo-controlled, multicenter studies designed to study the efficacy and safety of elinzanetant taken orally once daily in the treatment of women with moderate to severe VMS associated with menopause for 26 weeks. OASIS 1 and 2 randomly recruited 396 and 400 postmenopausal women aged 40-65 years at 184 sites in 15 countries.
OASIS 3 is a double-blind, randomized, placebo-controlled, multicenter study designed to study the efficacy and safety of elinzanetant in the treatment of vasomotor symptoms in postmenopausal women over 52 weeks. OASIS 3 randomized 628 postmenopausal women aged 40 to 65 years at 83 centers in 9 countries.
The results showed that elinzanetant met all primary endpoints in these three studies and showed a good safety profile.
In the OASIS-1 and OASIS-2 studies, elinzanetant significantly reduced the mean frequency and severity of moderate to severe VMS associated with menopause at weeks 4 and 12 compared with placebo. The efficacy was sustained through week 26, with more than 80% of subjects in the elinzanetant group experiencing at least a 50% reduction in VMS frequency, including patients who switched from placebo to elinzanetant after week 12.
In the OASIS-3 study, elinzanetant significantly reduced the mean frequency of moderate to severe VMS compared with placebo at week 12, and this efficacy was maintained throughout the study. OASIS-3 further confirmed the findings of OASIS-1 and OASIS-2, showing the sustained efficacy and safety of the therapy over 52 weeks.
The most commonly reported adverse reactions in the elinzanetant group were headache, fatigue, and somnolence.
Significance of approval and prospects
Menopausal symptoms, such as hot flashes, have a significant impact on women’s quality of life. These symptoms are not only physical discomfort, but can also seriously affect daily activities, sleep and emotional health. The approval of elinzanetant provides these patients with a non-hormonal treatment option that helps relieve symptoms and improve quality of life.
By 2030, the number of women experiencing menopause worldwide is expected to increase to 1.2 billion, with 47 million entering this stage each year. This large patient population provides broad space for the market potential of elinzanetant. With the approval of more countries and regions, elinzanetant is expected to benefit more patients worldwide.
The marketing application of elinzanetant is under review by regulatory authorities in other major markets such as the United States and the European Union. If approved, the drug will further consolidate Bayer’s position in women’s health and bring new growth points to the company.
Conclusion
The approval of Bayer’s elinzanetant is an important milestone in women’s health. This first-of-its-kind dual neurokinin targeted therapy brings new hope for the treatment of menopausal symptoms. It is not only the birth of a new drug, but also a reconstruction of the field of women’s health. Its significance goes far beyond hot flashes itself – it proves that precise neuromodulation can replace hormonal intervention and provide a safer and more lasting solution for 1.2 billion women.
With the accumulation of more clinical data and the advancement of market promotion, elinzanetant is expected to improve the quality of life of more women worldwide.
References
Pinkerton JV, Simon JA, Joffe H, Maki PM, Nappi RE, Panay N, Soares CN, Thurston RC, Caetano C, Haberland C, Haseli Mashhadi N, Krahn U, Mellinger U, Parke S, Seitz C, Zuurman L. Elinzanetant for the Treatment of Vasomotor Symptoms Associated With Menopause: OASIS 1 and 2 Randomized Clinical Trials. JAMA. 2024 Aug 22;332(16):1343–54. doi: 10.1001/jama.2024.14618. Epub ahead of print. PMID: 39172446; PMCID: PMC11342219.
https://jamanetwork.com/journals/jama/fullarticle/2822766
Simon JA, Anderson RA, Ballantyne E, Bolognese J, Caetano C, Joffe H, Kerr M, Panay N, Seitz C, Seymore S, Trower M, Zuurman L, Pawsey S. Efficacy and safety of elinzanetant, a selective neurokinin-1,3 receptor antagonist for vasomotor symptoms: a dose-finding clinical trial (SWITCH-1). Menopause. 2023 Mar 1;30(3):239-246. doi: 10.1097/GME.0000000000002138. Epub 2023 Jan 30. PMID: 36720081; PMCID: PMC9970022.
Sassarini J, Anderson RA. Elinzanetant: a phase III therapy for postmenopausal patients with vasomotor symptoms. Expert Opin Investig Drugs. 2024 Jan;33(1):19-26. doi: 10.1080/13543784.2024.2305122. Epub 2024 Feb 12. PMID: 38224099.
https://www.tandfonline.com/doi/full/10.1080/13543784.2024.2305122
Doggrell SA. Will elinzanetant, a neurokinin receptor antagonist, have a role in the treatment of hot flashes? Expert Opin Pharmacother. 2025 Mar;26(4):349-354. doi: 10.1080/14656566.2025.2465875. Epub 2025 Feb 15. PMID: 39927400.
https://www.tandfonline.com/doi/abs/10.1080/14656566.2025.2465875
Menegaz de Almeida A, Oliveira P, Lopes L, Leite M, Morbach V, Alves Kelly F, Barros Í, Aquino de Moraes FC, Prevedello A. Fezolinetant and Elinzanetant Therapy for Menopausal Women Experiencing Vasomotor Symptoms: A Systematic Review and Meta-analysis. Obstet Gynecol. 2025 Mar 1;145(3):253-261. doi: 10.1097/AOG.0000000000005812. Epub 2025 Jan 2. PMID: 39746208.
de Oliveira HM, Diaz CAV, Barbosa LM, Flávio-Reis VHP, Zamora FV, Gonçalves Barbosa Júnior O. Efficacy and safety of fezolinetant and elinzanetant for vasomotor symptoms in postmenopausal women: A systematic review and meta-analysis. Maturitas. 2025 Apr;195:108220. doi: 10.1016/j.maturitas.2025.108220. Epub 2025 Feb 22. PMID: 39987726.
https://www.sciencedirect.com/science/article/abs/pii/S0378512225000283




