Monoclonal Antibodies, the Latest Nature Series Review
Abstract
This review commemorates the 50-year evolution of monoclonal antibodies (mAbs), tracing their transformation from murine hybridoma-derived research tools to a central pillar of clinical medicine. The authors chronicle major milestones in antibody engineering, including chimeric, humanized, and fully human formats, and discuss how technological innovations have addressed immunogenicity, pharmacokinetics, and efficacy challenges. The expansion into bispecific antibodies, antibody–drug conjugates (ADCs), antibody fragments, and integration with cell therapies has broadened the therapeutic landscape across oncology, autoimmune diseases, and infectious diseases. The review also explores advances in drug delivery, especially the shift from intravenous to subcutaneous administration, and anticipates future breakthroughs through AI-guided design, conditionally active antibodies, and novel immunoglobulin isotypes. Despite remaining challenges, antibody therapeutics are poised to enter a new era of precision, modularity, and patient-centered delivery.
Introduction
Since Köhler and Milstein’s invention of hybridoma technology in 1975, monoclonal antibodies (mAbs) have progressed from basic laboratory tools to a cornerstone of modern clinical therapy. Over the past five decades, advances in antibody engineering, humanization, biomanufacturing, drug conjugation, and multispecific design have expanded their applications to oncology, autoimmune disorders, and infectious diseases. Today, more than 200 approved mAb-based products have transformed treatment landscapes and improved the lives of tens of millions of patients worldwide.
On August 7, 2025, Andrew C. Chan, Paul J. Carter, and Greg D. Martyn from the Antibody Engineering Department of Genentech in South San Francisco, USA, published a review article titled “Fifty years of monoclonals: the past, present and future of antibody therapeutics” in the journal Nature Reviews Immunology. With the theme of “50 Years of Monoclonal Antibodies”, the article systematically reviewed the key milestones of monoclonal antibodies from their inception to modern diversified development, and looked forward to possible future innovation directions.

Key Steps Leading to Antibodies as Therapeutic Drugs
This section traces the progression of monoclonal antibodies from their origins in mouse hybridoma technology to their role in clinical drug development. Early mouse-derived antibodies faced significant barriers in therapeutic use, including high immunogenicity, short circulation half-life, and limited effector functions. The introduction of chimeric, humanized, and fully human antibody technologies—combined with advances in biomanufacturing and preclinical evaluation—has steadily resolved these challenges, enabling monoclonal antibodies to become a mainstream therapeutic modality.
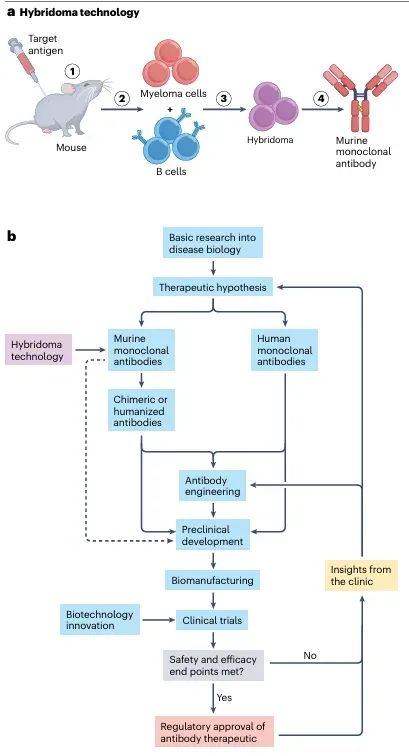
Figure 1: From hybridoma technology to antibody therapy.
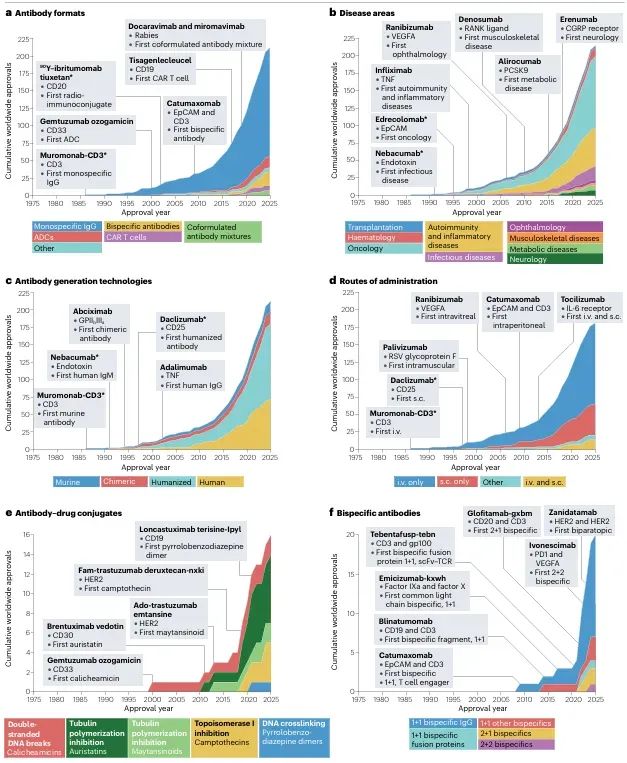
Figure 2: The rise of antibodies as therapeutics.
Evolution of Antibody Therapeutics
This section highlights the rapid expansion of antibody therapeutics in scale, indications, and molecular diversity. By 2025, a total of 212 monoclonal antibodies have gained global approval, addressing diseases across oncology, immunology, and infectious disease fields. Beyond conventional IgG antibodies, innovative formats such as antibody–drug conjugates (ADCs), bispecific antibodies, antibody fragments, fusion proteins, and CAR-T cell therapies are transforming the landscape. Administration routes have also diversified, evolving from solely intravenous infusion to include convenient subcutaneous delivery.
Advances in Antibody Technology Bring Improved Patient Benefits
This section examines B-cell–targeted therapies and HER2-positive cancers as case studies to demonstrate how antibody engineering can enhance both efficacy and safety. Key technological advances include glycoengineering to boost ADCC, bispecific antibodies to direct immune cell killing, antibody–drug conjugates for precise toxin delivery, and multimodal strategies integrated with cell therapies. Collectively, these innovations have extended patient survival and significantly improved quality of life.
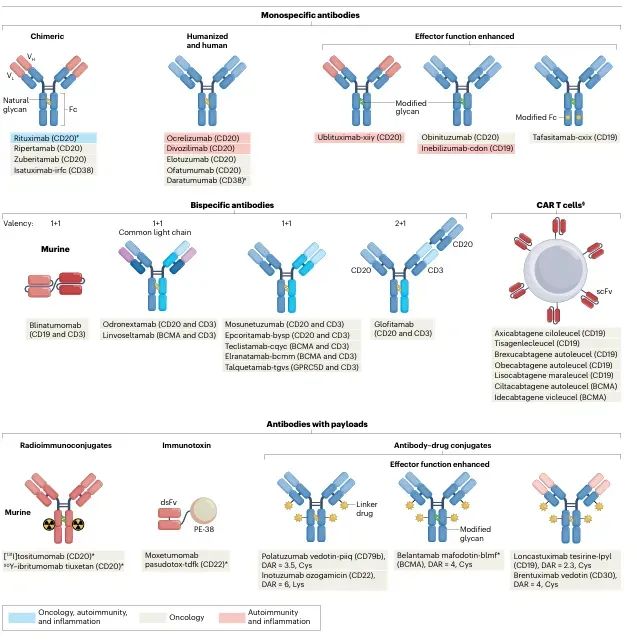
Figure 3: Antibody therapies targeting B cells in disease.
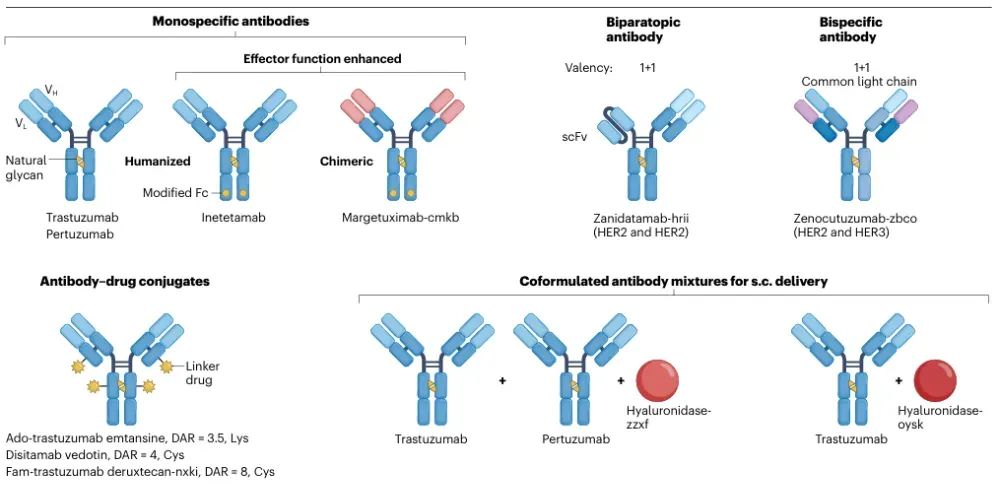
Figure 4: Antibody therapies targeting HER2 in oncology.
Current Status and Future Trends in Antibody Drug Delivery
The route of administration for antibody therapeutics plays a pivotal role in determining efficacy, patient convenience, and overall treatment cost. While intravenous infusion remains the predominant method, subcutaneous (SC) injection is rapidly gaining traction for its time efficiency and ability to ease the burden on infusion centers. Advances such as co-formulation with adjuvants like hyaluronidase now enable high-concentration SC delivery of large-molecule antibodies.
Looking ahead, emerging strategies include oral antibody delivery (overcoming gastrointestinal degradation and absorption challenges), targeted central nervous system administration (to traverse the blood–brain barrier), and long-acting sustained-release formulations (to prolong dosing intervals). These innovations aim to enhance treatment accessibility while improving patients’ quality of life.
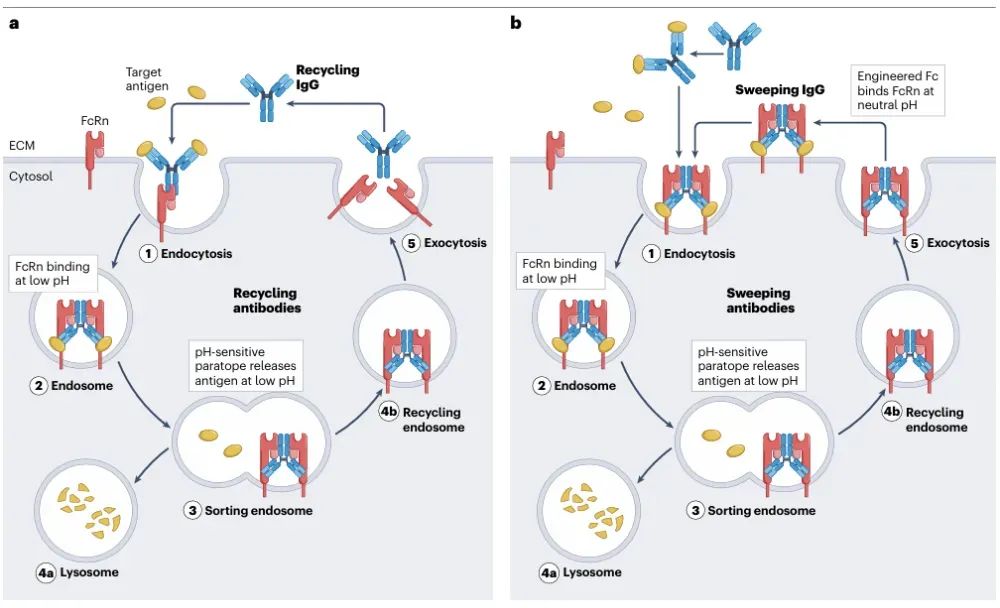
Figure 5: Recycling and scanning antibodies facilitate suprastoichiometric clearance of their cognate target antigens.
Future opportunities for antibody therapy
Future development directions include harnessing AI to accelerate antibody discovery and optimization, designing conditionally binding antibodies that activate only at targeted disease sites, investigating the clinical potential of novel immunoglobulin subtypes such as IgA and IgM, and integrating antibodies with cell therapies and RNA-based therapeutics to build multifunctional treatment platforms—enabling more precise, versatile, and effective disease interventions.
Summary and Outlook
The concluding section underscores the transformation of monoclonal antibodies from basic research tools into a cornerstone therapeutic class for a wide spectrum of diseases. Ongoing innovation is poised to deliver greater efficacy, fewer side effects, and increasingly personalized treatment options. However, challenges remain, including high production costs, resistance mechanisms, and immunogenicity risks. Looking ahead, advances in AI, synthetic biology, and next-generation manufacturing will be pivotal in driving the development of the next wave of antibody therapeutics.
Reference
Chan AC, Carter PJ, Martyn GD. Fifty years of monoclonals: the past, present and future of antibody therapeutics[J]. Nature Reviews Immunology, 2025.