Metformin - a Nice Drug for Various Diseases
Abstract
Metformin, a commonly used medication for type 2 diabetes, has been found to have potential therapeutic benefits in diseases beyond diabetes. Currently, metformin has demonstrated promising therapeutic potential in a range of diseases beyond diabetes, including PCOS, obesity, NAFLD, neurodegenerative diseases, gestational diabetes, and cancer-related metabolic syndrome, due to its effects on insulin sensitivity, glucose metabolism, inflammation, and cellular signaling pathways.
Metformin, a commonly used medication for type 2 diabetes, has been found to have potential therapeutic benefits in diseases beyond diabetes[1]. Metformin’s primary mechanism of action involves the activation of AMPK, which regulates cellular energy metabolism[2]. This mechanism leads to improved glucose utilization and lower blood glucose levels. In addition to its glucose-lowering effects, metformin has been shown to have pleiotropic effects on various cellular pathways, including inhibition of the mTOR pathway, which has been implicated in its potential anticancer effects[3]. Metformin has also been studied for its potential cardiovascular benefits, as it may improve lipid metabolism, reduce inflammation, and improve endothelial function[4]. Furthermore, metformin has been explored for its potential neuroprotective effects in neurodegenerative diseases, as well as its benefits in conditions such as polycystic ovary syndrome, obesity, and non-alcoholic fatty liver disease[5]. Further research is underway to better understand the mechanisms of action and optimize the clinical use of metformin in these diseases, potentially paving the way for new therapeutic applications of this widely used medication.
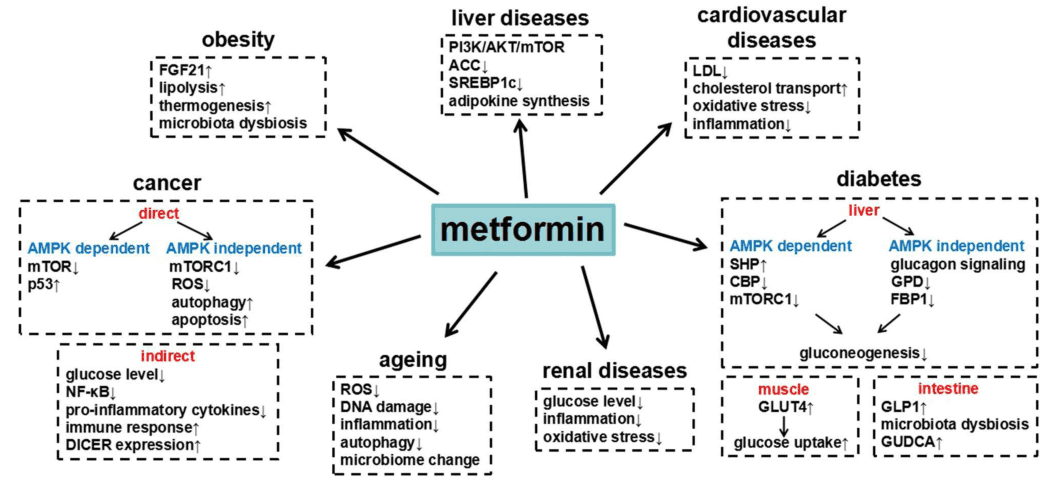
Figure 1 Summary of metformin in different diseases and the underlying major mechanisms.
Metformin and Cancer
Metformin has gained attention in recent years due to its potential anticancer effects. Extensive preclinical and clinical studies have investigated the role of metformin in cancer prevention and treatment, providing evidence for its potential benefits in various cancer types.
Metformin’s anticancer effects are thought to be mediated through multiple mechanisms[6]. One of the key mechanisms is the inhibition of the mammalian target of the rapamycin (mTOR) pathway, which plays a crucial role in regulating cell growth and proliferation. Metformin has been shown to suppress mTOR signaling, leading to the inhibition of cancer cell growth and proliferation.
Another important mechanism of metformin’s anticancer effects is the activation of AMP-activated protein kinase (AMPK), a cellular energy sensor that regulates energy metabolism. AMPK activation by metformin leads to the inhibition of cancer cell growth and proliferation, as well as induction of apoptosis (programmed cell death), and inhibition of angiogenesis (formation of new blood vessels that supply tumors)[7].
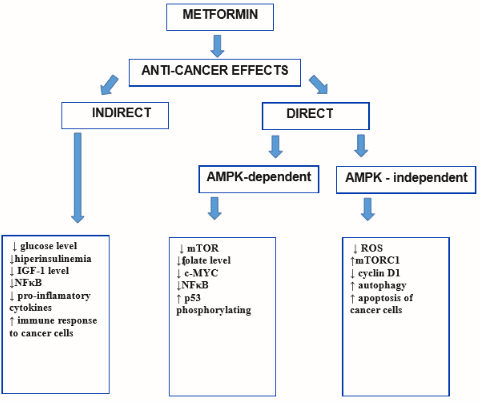
Figure 2 Anti-cancer effects of metformin[6]
In addition, metformin has been shown to modulate insulin signaling, which is implicated in cancer development and progression. Metformin can lower insulin levels and improve insulin sensitivity, which may help to reduce the risk of cancer development and improve outcomes in cancer patients.
Clinical evidence for metformin’s use in cancer prevention and treatment is emerging but still evolving. Several observational studies have suggested that metformin use is associated with a reduced risk of cancer incidence and improved cancer survival in patients with various cancer types, including breast, prostate, colorectal, and liver cancer. However, some studies have shown conflicting results, and more robust randomized controlled trials are needed to establish the true efficacy of metformin in cancer prevention and treatment.
Metformin has also been investigated as an adjunct to chemotherapy in cancer treatment. Some studies have shown that metformin can sensitize cancer cells to chemotherapy, leading to enhanced treatment response. However, the optimal timing, dosing, and patient selection for metformin as an adjunct to chemotherapy are still being explored.
Metformin and Cardiovascular Diseases
Metformin has been shown to have potential cardiovascular benefits beyond its glucose-lowering effects. Extensive research has investigated the effects of metformin on various cardiovascular risk factors and outcomes, including glycemic control, lipid metabolism, inflammation, and endothelial function.
One of the key cardiovascular benefits of metformin is its ability to improve glycemic control by reducing insulin resistance and lowering blood glucose levels. Better glycemic control has been associated with a reduced risk of cardiovascular diseases, such as myocardial infarction (heart attack), heart failure, and stroke. Metformin has also been shown to have favorable effects on lipid metabolism[8]. It can decrease LDL cholesterol (commonly known as “bad” cholesterol) levels and increase HDL cholesterol (often referred to as “good” cholesterol) levels, leading to improved lipid profiles. This may help reduce the risk of atherosclerosis, a major contributor to cardiovascular diseases. Inflammation plays a crucial role in the development and progression of cardiovascular diseases.
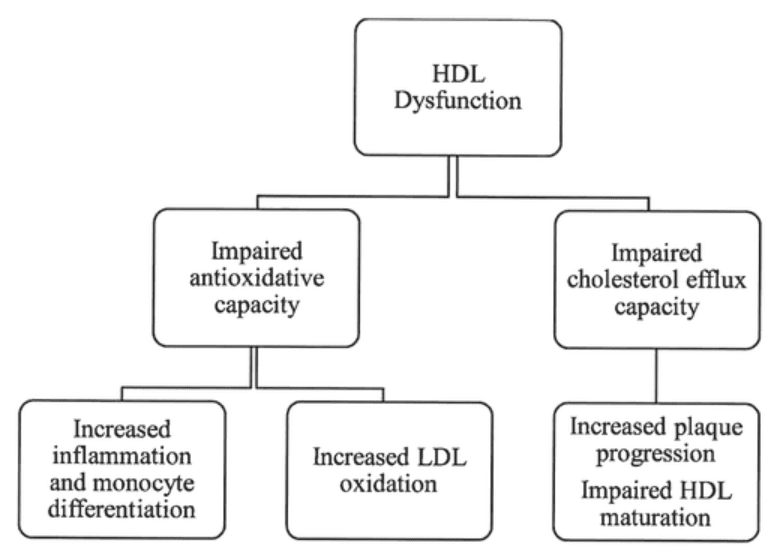
Figure 3 High-density lipoprotein dysfunction: clinical repercussions. HDL, high-density lipoprotein; LDL, low-density lipoprotein.[8]
Metformin has been shown to have anti-inflammatory effects, including reducing markers of inflammation such as C-reactive protein (CRP) and tumor necrosis factor-alpha (TNF-alpha). These anti-inflammatory effects may contribute to the cardiovascular benefits of metformin. Endothelial dysfunction, characterized by impaired endothelial function, is a hallmark of many cardiovascular diseases. Metformin has been shown to improve endothelial function by increasing the production of nitric oxide, a molecule that helps relax blood vessels and improve blood flow. This may help prevent or ameliorate endothelial dysfunction, thereby reducing the risk of cardiovascular diseases. Multiple research studies, including observational studies and randomized controlled trials, have presented compelling evidence supporting the cardiovascular advantages of metformin. Findings from observational studies have revealed that metformin usage is linked with a lowered risk of cardiovascular events, such as heart attacks and strokes, in individuals with diabetes. Moreover, randomized controlled trials have further supported these findings, demonstrating the favorable effects of metformin on cardiovascular outcomes, including reduced risk of heart failure and improved endothelial function. Metformin’s potential cardiovascular benefits may extend beyond patients with diabetes. Studies have shown that metformin may also have cardiovascular benefits in non-diabetic populations. For example, metformin has been shown to reduce the risk of cardiovascular events in obese individuals, even in the absence of diabetes. This suggests that metformin may have a broader role in preventing cardiovascular diseases beyond its use in diabetes management.
Metformin and Neurodegenerative Diseases
Metformin has been extensively investigated for its potential neuroprotective properties in neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease. Both preclinical and clinical studies have delved into the role of metformin in safeguarding against neurodegeneration and preserving cognitive function.
Metformin is believed to exert its neuroprotective effects through various mechanisms of action. One proposed mechanism is its impact on mitochondrial function. Metformin has been shown to enhance mitochondrial function, which plays a crucial role in cellular energy production and is often impaired in neurodegenerative diseases. By improving mitochondrial function, metformin may help shield neurons from damage and maintain their optimal functioning.
Metformin’s potential neuroprotective effects may also be attributed to its anti-inflammatory properties[9]. Neuroinflammation, characterized by chronic inflammation in the brain, is believed to be a significant factor in the development and progression of neurodegenerative diseases. Metformin has been shown to possess anti-inflammatory effects, including reducing markers of inflammation in the brain, which could contribute to its neuroprotective effects[10].
Furthermore, metformin’s impact on insulin signaling in the brain may play a role in its neuroprotective potential. Insulin resistance in the brain has been implicated in neurodegenerative diseases, and metformin has been shown to enhance insulin sensitivity. By improving insulin signaling in the brain, metformin may help safeguard against neurodegeneration and preserve cognitive function.
Clinical studies have yielded evidence supporting the potential neuroprotective effects of metformin in neurodegenerative diseases. Findings from clinical trials have shown that metformin usage is linked with improved cognitive function and reduced risk of developing Alzheimer’s disease in individuals with diabetes. Additionally, other studies have indicated potential benefits of metformin in Parkinson’s disease, including reduced motor symptoms and improved quality of life.
Despite the promising findings, there are limitations to the current research on metformin and neurodegenerative diseases. Most studies have been conducted in animal models or small-scale clinical trials, and results may not necessarily translate to all populations or disease stages. The optimal dosages, treatment duration, and timing of metformin use in neurodegenerative diseases remain unclear and require further investigation. Additionally, potential side effects and interactions with other medications need to be carefully considered in the context of neurodegenerative diseases.
Future research directions in studying metformin’s effects on neurodegenerative diseases may include larger and long-term clinical trials, investigating different populations, disease stages, and dosages of metformin. Mechanistic studies to further elucidate the underlying pathways by which metformin exerts its neuroprotective effects may also be warranted. Additionally, research exploring the potential combination of metformin with other therapies for neurodegenerative diseases may provide valuable insights into its efficacy and safety.
Metformin and Polycystic Ovary Syndrome (PCOS)
Numerous studies have examined the use of metformin as a supplementary treatment for the management of polycystic ovary syndrome (PCOS), a common endocrine disorder in women of childbearing age. PCOS is characterized by hormonal imbalances, insulin resistance, ovarian dysfunction, and irregular menstrual cycles. The potential advantages of metformin in enhancing insulin sensitivity, reviving ovarian function, normalizing menstrual cycles, and enhancing fertility results in females suffering from PCOS have been investigated.
One of the primary mechanisms through which metformin may exert its effects on PCOS is by improving insulin sensitivity. Insulin resistance is a hallmark of PCOS, resulting in elevated insulin levels and increased androgen production[11], which can disrupt ovarian function and menstrual cycles. Metformin has been shown to reduce insulin resistance and lower insulin levels, which may help restore normal ovarian function and regulate menstrual cycles in women with PCOS.
Research has demonstrated that metformin can directly impact ovarian function in women with PCOS. Specifically, metformin has been found to decrease the production of ovarian androgens, thereby restoring hormonal equilibrium[12]. This can enhance ovulation rates and boost the likelihood of pregnancy for women with PCOS who are attempting to conceive.
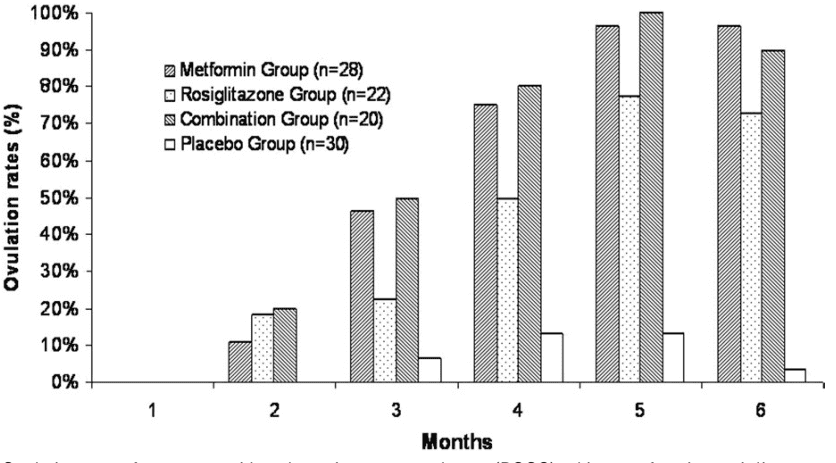
Figure 4 Ovulation rates for women with polycystic ovary syndrome (PCOS) taking metformin, rosiglitazone, combination, or placebo.[12]
Apart from its effects on insulin sensitivity and ovarian function, metformin may also have potential advantages on menstrual irregularities in females with PCOS. Metformin has been documented to regulate menstrual cycles and increase the frequency of ovulatory cycles, which can normalize menstrual patterns for women with PCOS who experience irregular or absent periods.
Additionally, investigations have explored the potential impact of metformin on fertility results in females with PCOS. According to studies, metformin may enhance fertility outcomes by increasing the likelihood of ovulation and pregnancy, as well as lowering the risk of pregnancy complications such as pre-eclampsia and gestational diabetes in PCOS patients.
Nonetheless, metformin has limitations in the management of PCOS, despite its potential benefits. The evidence of its effectiveness in enhancing fertility outcomes is mixed, with certain research indicating positive effects while others show no significant difference compared to alternative interventions. Furthermore, the optimal dose and duration of metformin treatment in PCOS are still unknown, and further research is required to establish standardized protocols.
Metformin and Other Diseases
Metformin has displayed potential advantages in diseases beyond diabetes. Investigations have explored its use in conditions such as obesity, non-alcoholic fatty liver disease (NAFLD), gestational diabetes, and cancer-related metabolic syndrome.
Obesity is a complex metabolic disorder that is linked to several health hazards, such as insulin resistance, cardiovascular diseases, and type 2 diabetes. Metformin has been investigated for its potential impacts on body weight reduction and metabolic parameters enhancement in people with obesity[13]. Certain studies have demonstrated that metformin may decrease body weight, body mass index (BMI), and waist circumference while enhancing insulin sensitivity in individuals with obesity. However, the evidence in this area is still limited, and further research is necessary to establish the safety and effectiveness of metformin as a weight loss intervention in obesity.
Non-alcoholic fatty liver disease (NAFLD) is a condition characterized by the accumulation of fat in the liver in the absence of excessive alcohol consumption[14]. Metformin has been investigated for its potential benefits in NAFLD due to its effects on insulin sensitivity, hepatic glucose production, and lipid metabolism. Some studies have shown that metformin may help reduce hepatic fat accumulation, improve liver function, and decrease inflammation in individuals with NAFLD. However, the evidence in this area is still evolving, and further research is needed to establish the role of metformin in the management of NAFLD.
Gestational diabetes mellitus (GDM) is a form of diabetes that occurs during pregnancy and can have negative consequences for both the mother and the fetus. Metformin has been investigated as a possible treatment alternative for GDM, with research indicating its efficacy in enhancing glycemic control throughout pregnancy. Metformin has been demonstrated to reduce blood glucose levels, minimize the necessity for insulin therapy, and enhance maternal and fetal outcomes in females with GDM. Nevertheless, the use of metformin in GDM must be cautiously assessed and managed by a competent healthcare professional.
A cancer-related metabolic syndrome is a group of metabolic abnormalities, such as obesity, insulin resistance, dyslipidemia, and hypertension, that are linked to an elevated risk of cancer. Metformin has been explored for its potential impact in alleviating cancer-related metabolic syndrome since it has been demonstrated to enhance insulin sensitivity, lipid metabolism, and blood pressure. Certain studies have suggested that metformin might play a role in decreasing the risk of cancer occurrence and enhancing cancer outcomes in persons with cancer-related metabolic syndrome. Nevertheless, further research is needed in this area to establish the safety and effectiveness of metformin as a therapeutic intervention in cancer-related metabolic syndrome.
Conclusion and Future Directions
In summary, metformin has demonstrated promising therapeutic potential in a range of diseases beyond diabetes, including PCOS, obesity, NAFLD, neurodegenerative diseases, gestational diabetes, and cancer-related metabolic syndrome, due to its effects on insulin sensitivity, glucose metabolism, inflammation, and cellular signaling pathways.
However, the current research has limitations, such as inconsistencies in study results, varying treatment durations and dosages, and differences in patient populations. Additionally, metformin may have side effects and contraindications that need to be carefully considered in different disease conditions and populations.
To further advance the understanding and potential applications of metformin, future research should focus on investigating the underlying mechanisms of its actions, exploring optimal dosages and treatment durations, identifying patient subpopulations that may benefit the most from metformin, and examining potential synergistic effects with other therapeutic interventions. Long-term safety and efficacy data are also needed to establish the role of metformin as a therapeutic option in various diseases.