Meet SKLB-11A: The New Molecule That Could Protect Your Heart and Mitochondria
Abstract
SKLB-11A is a novel small-molecule compound identified as a selective activator of Sirtuin 3 (SIRT3), a key mitochondrial enzyme involved in cellular energy regulation and oxidative stress reduction. Preclinical studies show that SKLB-11A enhances mitochondrial protein deacetylation, improves ATP production, and protects cardiomyocytes under stress conditions. Its targeted mechanism makes it a promising candidate for treating cardiovascular diseases, metabolic disorders, and age-related conditions. By addressing mitochondrial dysfunction at its core, SKLB-11A represents a significant advancement in epigenetic and metabolic medicine. Ongoing research continues to explore its therapeutic potential, with future clinical trials anticipated to assess its efficacy and safety in humans.
Introduction: What is SKLB-11A?
In the rapidly evolving field of biomedical research, one molecule is drawing increasing attention for its potential in combating age-related diseases and cardiovascular dysfunction—SKLB-11A. Classified as a small-molecule compound, SKLB-11A has emerged as a promising activator of Sirtuin 3 (SIRT3), a mitochondrial enzyme involved in cellular metabolism, oxidative stress reduction, and longevity regulation.
Discovered through targeted compound screening, SKLB-11A is designed to enhance SIRT3 activity, which in turn supports healthy mitochondrial function. Mitochondria are essential for energy production and cellular homeostasis, but they also play a pivotal role in the aging process and disease progression. By activating SIRT3, SKLB-11A may help reduce mitochondrial protein acetylation, leading to improved energy metabolism and cellular resilience.
What sets SKLB-11A apart from earlier sirtuin activators is its distinct binding mechanism, enabling it to modulate SIRT3 more effectively. Preclinical studies have demonstrated that SKLB-11A enhances mitochondrial efficiency and offers cardioprotective benefits, particularly in models of oxidative stress and ischemia.
As researchers continue to explore the therapeutic implications of SIRT3 modulation, SKLB-11A stands at the forefront of a new class of compounds aimed at targeting mitochondrial health. With potential applications in cardiovascular disease, metabolic disorders, and age-related conditions, SKLB-11A could become a key player in next-generation medical treatments.
The Science Behind SKLB-11A: How It Works
SKLB-11A owes its therapeutic promise to its unique ability to activate Sirtuin 3 (SIRT3), a key regulator of mitochondrial function. SIRT3 is a member of the sirtuin family of NAD⁺-dependent deacetylases and plays a vital role in controlling oxidative stress, energy metabolism, and mitochondrial integrity.
Under normal conditions, SIRT3 helps maintain mitochondrial health by removing acetyl groups from mitochondrial proteins, thereby enhancing their function. However, in states of aging, metabolic dysfunction, or oxidative stress, SIRT3 activity often declines—leading to excessive protein acetylation, reduced energy output, and increased cellular damage.

This is where SKLB-11A comes in. Unlike traditional activators that may have broad or nonspecific activity, SKLB-11A binds to a distinct allosteric site on the SIRT3 enzyme. This targeted interaction not only boosts enzymatic activity but does so without interfering with other sirtuin family members, minimizing off-target effects.
In preclinical models, especially in H9c2 cardiomyocyte cells, SKLB-11A has been shown to enhance mitochondrial deacetylation levels, improve ATP production, and reduce reactive oxygen species (ROS). These effects point to a robust cardioprotective mechanism, making SKLB-11A a strong candidate for treating conditions like ischemic heart disease, heart failure, and metabolic syndrome.
Importantly, SKLB-11A operates at the intersection of epigenetics and metabolism—a growing area in drug development known as “mitochondrial medicine.” As scientists uncover more about how metabolic enzymes regulate gene expression and cell survival, compounds like SKLB-11A are expected to play a central role in future therapies.
Preclinical Research and Therapeutic Potential of SKLB-11A
Recent breakthroughs in preclinical research have shed light on the transformative therapeutic potential of SKLB-11A, particularly in the realm of cardiovascular and mitochondrial diseases. As a selective activator of SIRT3, SKLB-11A has demonstrated the ability to enhance mitochondrial function and reduce oxidative stress—two crucial mechanisms in preventing and treating cardiac injury.
A 2025 study published in ACS Central Science by Zhang et al. marked a pivotal milestone. In this study, SKLB-11A significantly boosted SIRT3 activity in H9c2 cardiomyocyte cells, leading to a decrease in acetylated mitochondrial proteins, restoration of ATP levels, and reduction of reactive oxygen species (ROS). These effects collectively point to improved mitochondrial efficiency and resilience against oxidative damage.
Moreover, SKLB-11A was shown to protect cardiomyocytes under conditions mimicking ischemia-reperfusion injury, a major contributor to heart attack-related damage. By activating SIRT3 and preserving mitochondrial integrity, SKLB-11A may offer a novel therapeutic strategy for heart failure, myocardial infarction, and cardiometabolic disorders.
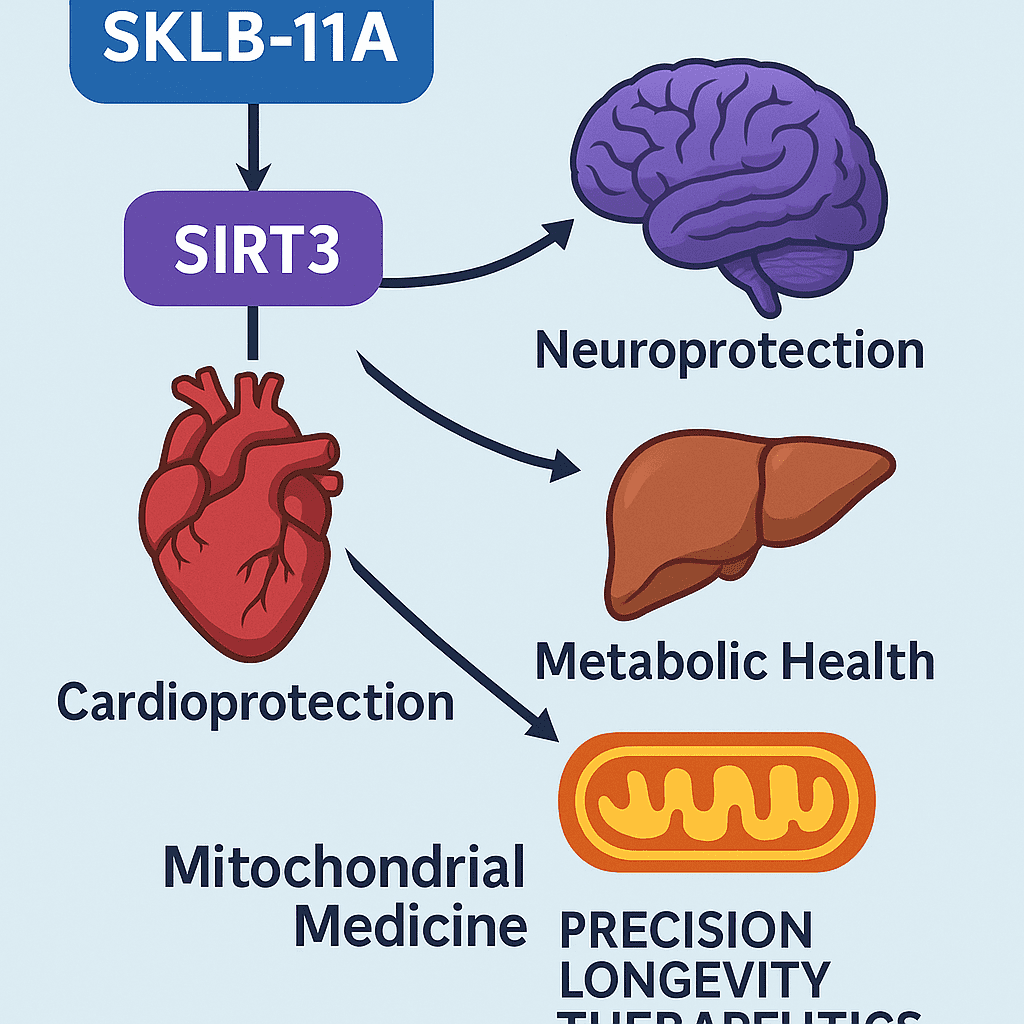
Beyond the heart, SIRT3 plays key roles in tissues such as the brain, liver, and kidneys. Researchers are now investigating SKLB-11A’s potential in treating neurodegenerative diseases, metabolic syndrome, and even age-related disorders, where mitochondrial dysfunction is a known driver.
Importantly, SKLB-11A’s target specificity and favorable safety profile in preclinical models make it a promising candidate for further drug development. Clinical trials will be the next step to evaluate its pharmacokinetics, toxicity, and efficacy in humans.
As interest in mitochondrial medicine grows, SKLB-11A stands out as one of the most compelling experimental drugs currently under investigation.
Why SKLB-11A Matters: Implications for Future Medicine
In an era where chronic diseases like heart failure, neurodegeneration, and metabolic syndrome are on the rise, the discovery of SKLB-11A marks a significant leap forward in precision mitochondrial medicine. By selectively activating Sirtuin 3 (SIRT3), SKLB-11A addresses one of the most fundamental drivers of cellular decline: mitochondrial dysfunction.
What makes SKLB-11A especially promising is its targeted, allosteric modulation of SIRT3—unlike other sirtuin activators that act non-specifically across multiple pathways. This specificity not only increases therapeutic efficacy but also reduces the likelihood of unwanted side effects, a critical consideration for long-term use in chronic conditions.
As research continues, the role of SIRT3 in various organs is becoming more apparent. In the brain, for instance, SIRT3 regulates oxidative stress and mitochondrial dynamics—factors implicated in Alzheimer’s and Parkinson’s disease. In the liver, SIRT3 modulates lipid metabolism, positioning SKLB-11A as a candidate for non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes treatment.
Furthermore, SKLB-11A represents a paradigm shift toward epigenetic-metabolic drug development—a cutting-edge intersection of metabolic regulation and gene expression control. This innovative approach holds potential for multi-system benefits, particularly in age-related diseases that share mitochondrial pathology.
As the biotech and pharmaceutical industries intensify their focus on longevity therapeutics, compounds like SKLB-11A will likely shape the future of personalized medicine. While clinical validation is still pending, the scientific foundation already makes a compelling case for SKLB-11A as a next-generation mitochondrial modulator.
Final Thoughts and Future Directions for SKLB-11A
SKLB-11A is more than just a novel compound—it’s a potential game-changer in the world of mitochondrial medicine. With its unique ability to selectively activate Sirtuin 3 (SIRT3), this small molecule opens new doors in the treatment of cardiovascular disease, metabolic dysfunction, and possibly even age-related neurodegeneration.
So far, preclinical studies have painted an encouraging picture: improved mitochondrial function, reduced oxidative stress, and enhanced cellular resilience. These benefits make SKLB-11A a strong candidate for tackling chronic diseases at the root cause—mitochondrial decline.
However, much work remains. While its safety profile and target specificity are promising, SKLB-11A has not yet entered clinical trials. Researchers must now validate its efficacy in animal models, evaluate its pharmacokinetics and toxicity, and eventually move toward human testing.
The broader implication? SKLB-11A could be one of the first of many next-generation drugs that treat diseases not by masking symptoms, but by optimizing cellular performance from within. As scientific interest in NAD⁺ metabolism, sirtuins, and mitochondrial health continues to grow, SKLB-11A will likely be part of a larger wave of longevity-focused therapeutics.
For now, staying informed about ongoing research and future trial results will be key. Whether you’re a healthcare professional, biotech investor, or simply curious about the future of medicine, SKLB-11A is a name worth remembering.
References
Zhang, D., Zhang, J., Wu, C., Xiao, Y., Ji, L., Hu, J., et al. (2025). Unraveling small molecule-mediated Sirtuin 3 activation at a distinct binding site for cardioprotective therapies. ACS Central Science.
https://pubs.acs.org/doi/full/10.1021/acscentsci.5c00023
Hirschey, M. D., Shimazu, T., Goetzman, E., Jing, E., Schwer, B., Lombard, D. B., … & Verdin, E. (2010). SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature, 464(7285), 121-125.
https://www.nature.com/articles/nature08778
Lombard, D. B., Alt, F. W., Cheng, H. L., Bunkenborg, J., Streeper, R. S., Mostoslavsky, R., … & Guarente, L. (2007). Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Molecular and Cellular Biology, 27(24), 8807-8814.
https://www.tandfonline.com/doi/abs/10.1128/MCB.01636-07
Someya, S., Yu, W., Hallows, W. C., Xu, J., Vann, J. M., Leeuwenburgh, C., … & Prolla, T. A. (2010). Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell, 143(5), 802-812.
https://www.cell.com/fulltext/S0092-8674(10)01138-4
Ansari, A., Rahman, M. S., Saha, S. K., Saikot, F. K., Deep, A., & Kim, K. H. (2017). Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell, 16(1), 4–16.
https://onlinelibrary.wiley.com/doi/full/10.1111/acel.12538
Sundaresan, N. R., Gupta, M., Kim, G., Rajamohan, S. B., Isbatan, A., & Gupta, M. P. (2009). SIRT3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. Journal of Clinical Investigation, 119(9), 2758–2771.
https://www.jci.org/articles/view/39162
Rajendran, R., Garva, R., Krstic-Demonacos, M., & Demonacos, C. (2011). Sirtuins: molecular traffic lights in the crossroad of oxidative stress, chromatin remodeling, and transcription. Journal of Biomedicine and Biotechnology, 2011.
https://onlinelibrary.wiley.com/doi/full/10.1155/2011/368276
Choudhary, C., Weinert, B. T., Nishida, Y., Verdin, E., & Mann, M. (2014). The growing landscape of lysine acetylation links metabolism and cell signalling. Nature Reviews Molecular Cell Biology, 15(8), 536–550.