Mechanisms and Therapeutic Advances in Cancer Metastasis: From Biological Pathways to Clinical Strategies
Abstract
Cancer metastasis is a leading cause of cancer-related mortality, involving the spread of malignant cells from the primary tumor to distant organs, forming resistant secondary growths. This review examines the biological mechanisms of metastasis, which unfolds in key steps: invasion, circulation, colonization, and proliferation. Key processes, including epithelial-mesenchymal transition (EMT), circulatory survival, and organ-specific colonization, are critical for metastatic spread. The review also explores the role of signaling pathways, tumor microenvironment interactions, and chromosomal instability in metastasis. Recent therapeutic advancements targeting circulating tumor cells (CTCs), enhancing immunotherapies, and preventing recurrence are highlighted, along with strategies for patient management through early screening and lifestyle adjustments.
Cancer is frightening not only because of the destructive power of the primary tumor, but also because of its ability to metastasize – cancer cells “escape” from the primary site, spread throughout the body and form new lesions, ultimately leading to the death of more than 90% of cancer patients. Understanding this process is a key step in conquering cancer. This issue will start from the biological mechanism of cancer cell metastasis and combine the latest research progress to reveal the “secret path” of cancer spread.
Definition and clinical significance of metastasis
Metastasis is the process by which cancer cells break away from the primary tumor, migrate to other organs through the blood or lymphatic system, and form new tumors. For example, breast cancer often metastasizes to the bones, lungs, and liver; colorectal cancer easily metastasizes to the liver; and lung cancer may spread to the brain.
Key data:
About 70% of cancer patients have micrometastases when diagnosed.
The 5-year survival rate of patients with metastatic cancer is only 21%, far lower than the 89% of patients with localized cancer.
Clinical challenges:
Metastatic lesions are often insensitive to traditional chemotherapy and radiotherapy, and may suddenly recur after a long period of “dormancy”, which becomes a difficult point for treatment.
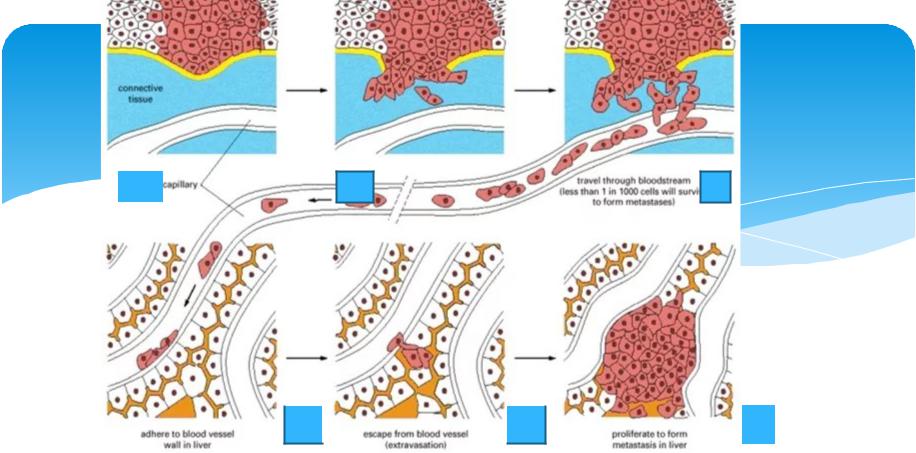
The “Four Steps” of Cancer Cell Metastasis
Cancer cell metastasis is not a random event, but a precisely regulated biological process, which can be divided into the following four key steps:
1. Invasion and shedding: from “keeping to oneself” to “armed breakthrough”
Epithelial-mesenchymal transformation (EMT):
Cancer cells lose their original adhesion through EMT and gain migration ability. This process is similar to “metamorphosis” – epithelial cells transform into mesenchymal cells, which are more flexible in morphology and can secrete proteases (such as MMPs) to degrade the basement membrane.
Microenvironment assistance:
Fibroblasts and immune cells around the tumor secrete growth factors (such as TGF-β) to promote cancer cell invasion.
2. Entering the circulatory system: the survival test of “hitchhiking”
Cancer cells that enter the blood are called circulating tumor cells (CTCs), but only 0.01% can survive. Blood flow shear force and immune attacks (such as NK cells) are the main threats.
Latest discovery: The team from the University of Macau found that cancer cells with high expression of desmosomal proteins (DSC2 and PKP1) can form cell clusters to resist blood flow pressure, and the survival rate is increased by 5 times.
3. Distant colonization: looking for a “new home”
Organ specificity:
Cancer cells recognize adhesion molecules of target organs through surface receptors (such as integrins). For example, the CXCR4 receptor on the surface of breast cancer cells binds to the CXCL12 chemokine in lung tissue, promoting lung metastasis.
“Seed and soil” hypothesis:
The formation of metastatic lesions depends on the matching of cancer cells (seeds) and the microenvironment of the target organ (soil). Brain metastases often rely on neural-like metabolic programs to adapt to the brain microenvironment.
4. Proliferation and angiogenesis: establishing a “colonial stronghold”
After colonization, cancer cells induce new blood vessels by secreting vascular endothelial growth factor (VEGF) to obtain nutritional support.
Dormancy and awakening: Some cancer cells can remain dormant for a long time until inflammation, hormonal changes or gene mutations trigger proliferation.
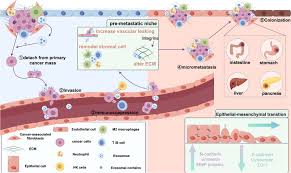
The “black hands behind the scenes” that drive metastasis
Every step of cancer cell metastasis is regulated by specific molecular mechanisms. The following are the key factors revealed by recent studies:
1. Signaling pathway: cascade reaction of AKT3, AXL and TBK1
AKT3: promotes cancer cell survival and drug resistance. Its high expression is positively correlated with the risk of breast cancer and lung cancer metastasis. The latest clinical trials show that AKT3 inhibitors can reduce the rate of distant metastasis.
AXL-TBK1 pathway: regulates EMT and immune escape. Blocking this pathway can inhibit cancer cell invasion.
2. Tumor microenvironment: immunosuppression and interstitial remodeling
Immune escape: In metastatic lesions, immunosuppressive myeloid cells (such as M2 macrophages) inhibit T cell activity through molecules such as PD-L1 and IL-10.
Interstitial fibroblasts: secrete collagen to form a physical barrier to protect cancer cells from drug attacks.
3. Chromosomal instability: the driving force of malignant evolution
Single-cell sequencing shows that metastatic cancer cells have higher chromosomal instability, leading to gene copy number variation and drug resistance.
Latest progress: from mechanism to therapeutic breakthrough
1. New strategy for targeting circulating tumor cells
DSC2/PKP1 inhibitors: The team from the University of Macau found that inhibiting these two proteins can destroy cancer cell clusters and reduce the risk of metastasis.
Liquid biopsy technology: By detecting ctDNA in the blood, early warning of metastasis recurrence can be given (sensitivity of 95%).
2. Optimization of immunotherapy
“Smart police force”: PD-1 inhibitors combined with TIL therapy can activate T cells to accurately eliminate metastatic lesions. In esophageal cancer, this regimen reduced tumor size in 24% of patients.
3. Preventive intervention
STING pathway agonists: Activate the innate immune system and eliminate dormant cancer cells. Phase I trials showed that the risk of recurrence in pancreatic cancer patients was reduced by 40%.
Patient action recommendations
1. Early screening: Regular testing of biomarkers such as AKT3 and ctDNA in high-risk groups.
2. Lifestyle: Anti-inflammatory diet (such as fish rich in Omega-3), regular exercise (enhancing NK cell activity).
3. Participation in clinical trials: Focus on innovative therapies targeting AKT3 and DSC2/PKP1 (such as NCT04697628).
References
Guo X, Chen C, Zhao J, Wang C, Mei X, Shen J, Lv H, Han Y, Wang Q, Lv J, Chen H, Yan X, Liu Z, Zhang Z, Zhong Q, Jiang Y, Xu L, Li X, Qian D, Ma D, Ye M, Wang C, Wang Z, Lin J, Tian Z, Leng X, Li Z. Neoadjuvant Chemoradiotherapy vs Chemoimmunotherapy for Esophageal Squamous Cell Carcinoma. JAMA Surg. 2025 Mar 19:e250220. doi: 10.1001/jamasurg.2025.0220. Epub ahead of print. PMID: 40105813; PMCID: PMC11923775.
https://jamanetwork.com/journals/jamasurgery/fullarticle/2831576
Xing X, Zhong J, Biermann J, Duan H, Zhang X, Shi Y, Gao Y, He K, Zhai D, Luo F, Lai Y, Xiao F, Wang W, Wang M, Xu J, Liu H, Tang J, Chu L, Chen T, D’Souza EK, Caprio L, Ebel L, Biswas D, Cottarelli A, Mou Y, Izar B, Zhang N, Bai F. Pan-cancer human brain metastases atlas at single-cell resolution. Cancer Cell. 2025 Apr 7:S1535-6108(25)00126-6. doi: 10.1016/j.ccell.2025.03.025. Epub ahead of print. PMID: 40215980.
https://www.cell.com/cancer-cell/abstract/S1535-6108(25)00126-6




