Mcl-1, a Target for Cancer Treatment
Abstract
Hematologic malignancies are a group of cancers that harm the blood, bone marrow, and lymphatic system. They result from the growth of abnormal cells, which can cause harm to healthy blood cells and compromise the immune system. Hematologic malignancies comprise various cancer types, such as leukemia, lymphoma, and multiple myeloma, each with unique symptoms, characteristics, and treatment alternatives[1].
Overview of hematologic malignancies
Leukemia is a cancer type that primarily targets the blood and bone marrow. It is identified by the propagation of immature white blood cells that can accumulate in these areas, causing anemia, weakness, fatigue, and a heightened susceptibility to infection. Acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), and chronic myeloid leukemia (CML) are the different types of leukemia. Identifying the specific type of leukemia is crucial in devising a suitable treatment plan.
Lymphoma is a form of cancer that impacts the lymphatic system, which encompasses the lymph nodes, spleen, thymus, and bone marrow[2]. It is distinguished by the atypical growth of lymphocytes, a type of white blood cell that can generate tumors in the lymphatic system and other organs. Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) are the two primary types of lymphoma, each with distinct subtypes and treatment methods.
Multiple myeloma is a type of cancer that affects plasma cells, a white blood cell variety that creates antibodies. It is characterized by the unusual growth and accumulation of plasma cells in the bone marrow, leading to bone pain, fractures, anemia, and an increased susceptibility to infection.
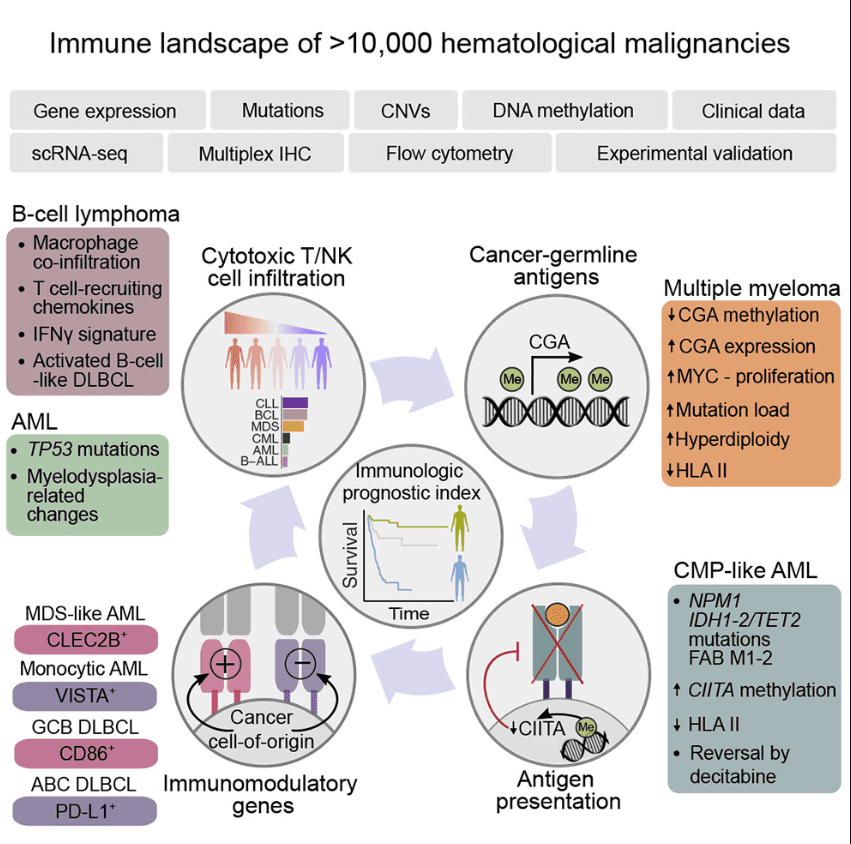
Figure 1 Immunogenomic Landscape of Hematological Malignancies[3]
Although the root causes of hematologic malignancies are not fully understood, it is believed that they are the result of a combination of genetic, environmental, and lifestyle factors. Certain risk factors for these cancers include exposure to radiation or particular chemicals, infection with certain viruses or bacteria, and a family history of cancer.
The course of treatment for hematologic malignancies is dependent on various factors, including the cancer type and stage, the patient’s age, overall health, and other individual considerations. Treatment options may include chemotherapy, radiation therapy, targeted therapy, immunotherapy, or stem cell transplantation. The ultimate aim of treatment is to eradicate as much of the cancer as possible, while also minimizing side effects and preserving normal blood cell function.
In summary, hematologic malignancies are a multifaceted and diverse category of cancers that affect the blood, bone marrow, and lymphatic system. These cancers can present with a variety of symptoms and may pose difficulties in diagnosis and treatment. Although advances in research and treatment have resulted in improved outcomes for many individuals with hematologic malignancies, additional research is necessary to create more effective therapies and enhance the quality of life for those impacted by these cancers.
Importance of MCL-1 in cell survival and cancer
MCL-1 (myeloid cell leukemia-1) is a protein that plays a critical role in regulating cell survival and apoptosis (programmed cell death). MCL-1 belongs to the BCL-2 family of proteins, which are involved in the regulation of apoptosis by controlling the permeability of the mitochondrial outer membrane. MCL-1 is a pro-survival member of this family, which means it promotes cell survival by inhibiting apoptosis(Figure 2).
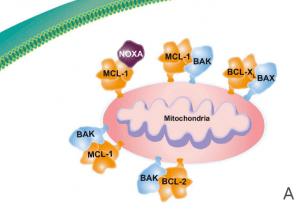
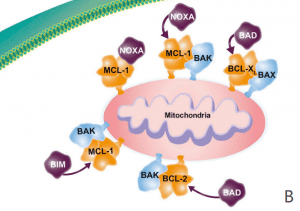
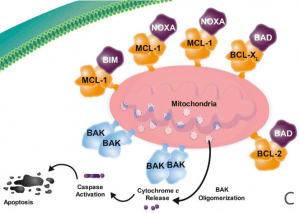
Figure 2 Overview of the role of MCL-1. (A) The antiapoptotic proteins (e.g. BCL-2, BCL-XL, BCL-W, MCL-1) are prosurvival proteins that bind the proapoptotic multidomain effectors BAK and BAX to prevent cell death, promoting cell survival. (B) A variety of cell stressors increase the expression of the proapoptotic sensors, including the BH3-only proteins (i.e., BIM, BID, PUMA, NOXA, and BAD). (C) The BH3-only proteins subsequently displace or prevent the antiapoptotic proteins from binding to BAX and BAK, leading to cytochrome c release into the cytosol and activation of the caspase cascade, resulting in cell death. BCL-2 = B-cell lymphoma–2; BH3 = BCL-2 homology 3; MCL-1 = myeloid cell leukemia sequence 1.[4]
The importance of MCL-1 in cancer lies in its ability to promote the survival of cancer cells, even in the face of chemotherapy or other stressors that would normally trigger apoptosis[5]. This is because cancer cells often have high levels of MCL-1 expression, which helps them evade the body’s natural defenses against cancer. In fact, MCL-1 is overexpressed in many different types of cancer, including hematologic malignancies (e.g. leukemia, lymphoma, multiple myeloma) and solid tumors (e.g. breast cancer, lung cancer, colon cancer).[6-8]
Targeting MCL-1 has become an attractive strategy for cancer therapy due to its role in promoting cancer cell survival and resistance to chemotherapy. Inhibiting MCL-1 may induce apoptosis in cancer cells and overcome resistance to treatment. Various approaches have been developed to target MCL-1 in cancer, including small molecule inhibitors, BH3-mimetics, and RNA interference. Small molecule inhibitors have shown promise in preclinical studies, but their development has been limited by challenges related to specificity and pharmacokinetics. BH3-mimetics are a class of drugs that mimic the function of BH3-only proteins, which are endogenous inhibitors of BCL-2 family pro-survival proteins like MCL-1. By mimicking the BH3-only proteins, BH3-mimetics can bind to and inhibit MCL-1, triggering apoptosis in cancer cells. Several BH3-mimetics are currently in clinical development for the treatment of hematologic malignancies and solid tumors. RNA interference is a technique for silencing specific genes by targeting their messenger RNA (mRNA) for degradation. Several approaches to RNA interference targeting MCL-1 have been developed, including small interfering RNA (siRNA) and short hairpin RNA (shRNA). While RNA interference holds promise as a therapeutic approach for cancer, it also presents challenges related to delivery and specificity. Nevertheless, the development of MCL-1-targeted therapies represents a significant area of research in cancer treatment with the potential to improve outcomes for cancer patients.
MCL-1 biology
A. MCL-1 structure and function
MCL-1 is a protein that plays a crucial role in cell survival and is encoded by the MCL1 gene located on chromosome 1(Figure 3). The MCL1 gene contains 10 exons, and the protein product of this gene is a 350 amino acid protein with a molecular weight of approximately 37 kDa. MCL-1 is a member of the BCL-2 family of proteins that regulate apoptosis by controlling the permeability of the mitochondrial outer membrane. As a pro-survival protein, MCL-1 inhibits apoptosis and promotes cell survival.
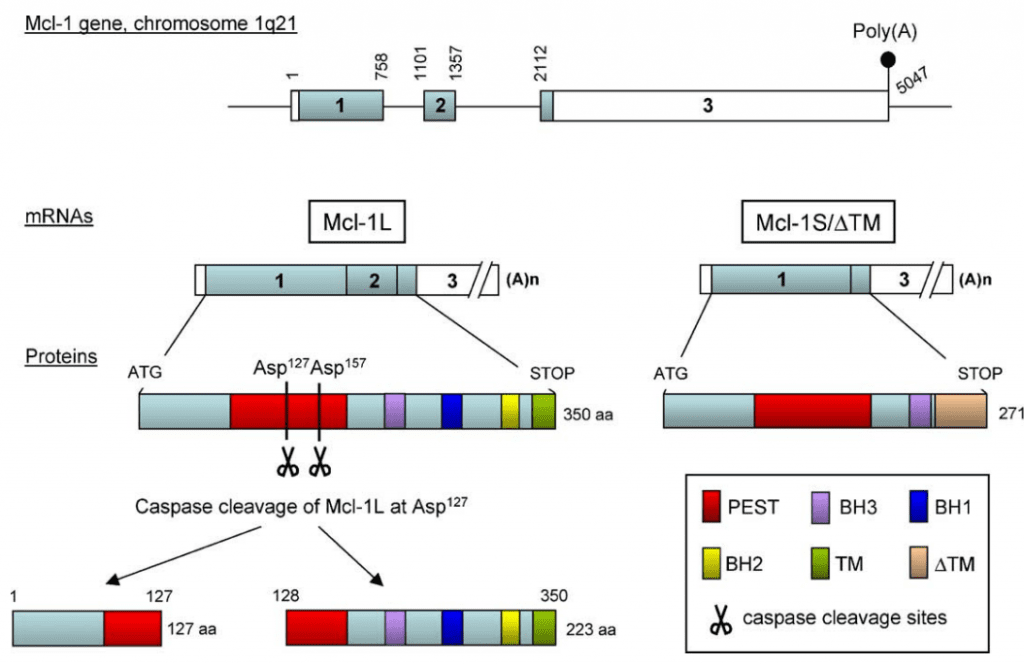
Figure 3 Molecular organization of Mcl-1[9]
MCL-1’s structure consists of three domains: the N-terminal domain, the central domain, and the C-terminal domain. The N-terminal domain facilitates dimerization, while the central domain contains the BH1, BH2, and BH3 domains. The BH3 domain is the site where MCL-1 interacts with other members of the BCL-2 family, including pro-apoptotic BH3-only proteins. The C-terminal domain anchors MCL-1 to the mitochondrial membrane. Understanding the structure and function of MCL-1 is essential in developing strategies to target this protein for cancer therapy.
B. Regulation of MCL-1 expression
MCL-1 expression is regulated at multiple levels, including transcriptional, post-transcriptional, and post-translational mechanisms. The promoter region of the MCL1 gene contains binding sites for multiple transcription factors, including c-Myc, NF-κB, and STAT3. Activation of these transcription factors can lead to increased MCL-1 expression.
Post-transcriptional regulation of MCL-1 expression is mediated by microRNAs (miRNAs). Several miRNAs have been identified that target the 3′ untranslated region (UTR) of the MCL1 mRNA, leading to its degradation and decreased protein expression. In addition, MCL-1 protein stability is regulated by post-translational modifications, including phosphorylation, ubiquitination, and proteasomal degradation.
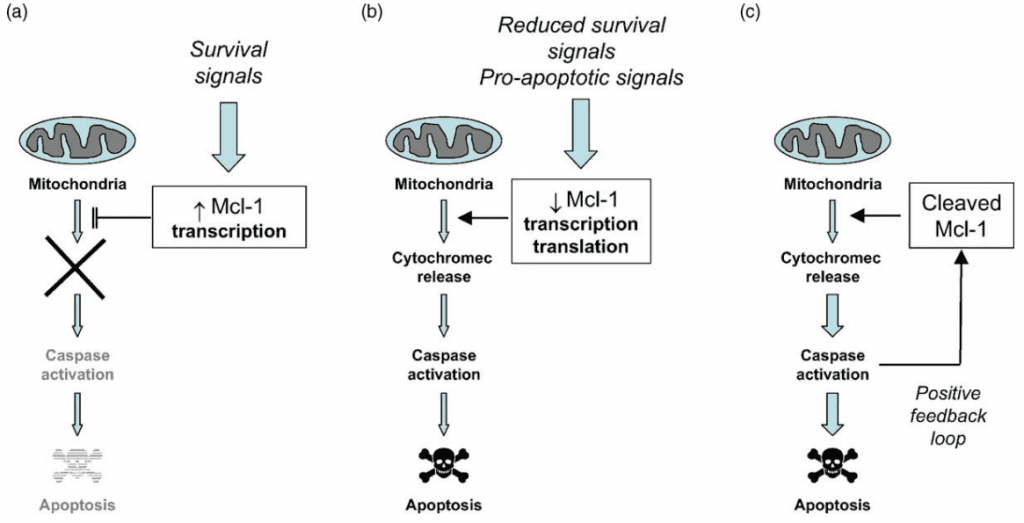
Figure 4 Regulation of Apoptosis by Mcl-1. (a) Induction of Mcl-1 expression by survival signals may contribute to resistance to apoptosis. (b) Rapid downregulation of Mcl-1 expression following removal of survival factors or exposure to other pro-apoptotic signals may contribute to apoptosis by promoting cytochrome c release. (c) Caspases activated during apoptosis can cleave remaining Mcl-1, generating a potent cell death promoting protein.[9]
C. Role of MCL-1 in hematologic malignancies
MCL-1 is a crucial protein for the survival of both normal and cancerous hematopoietic cells. In healthy hematopoietic cells, MCL-1 is essential for the survival of various cell types, including lymphocytes, erythrocytes, and platelets, as well as hematopoietic stem and progenitor cells. However, in hematologic malignancies, MCL-1 is often overexpressed and plays a critical role in promoting cancer cell survival and chemotherapy resistance. For instance, in acute myeloid leukemia (AML), MCL-1 is upregulated in approximately 80% of cases and associated with poor prognosis. Similarly, in chronic lymphocytic leukemia (CLL), MCL-1 expression is increased after chemotherapy, suggesting its involvement in mediating therapy resistance. Overall, targeting MCL-1 represents an essential therapeutic strategy to combat hematologic malignancies by inducing apoptosis in cancerous cells and overcoming resistance to chemotherapy.
MCL-1 is a crucial protein that regulates cell survival and apoptosis, and it is often overexpressed in hematologic malignancies. Its high expression in multiple myeloma cells has been found to mediate resistance to therapy, particularly proteasome inhibitors. Inhibition of MCL-1 can lead to apoptosis in these cells, making it a promising target for new cancer therapies. Several approaches are currently being studied to target MCL-1, and research in this area is ongoing. Overall, MCL-1’s role in cell survival and apoptosis regulation highlights its potential importance in cancer therapy, particularly in hematologic malignancies where it is often overexpressed.
How to treat cancer targeting MCL-1?
A. Rationale for MCL-1 inhibition:
MCL-1 is a key anti-apoptotic protein that plays a critical role in regulating cell survival and apoptosis in cancer cells. Its overexpression is associated with resistance to chemotherapy and poor prognosis in various types of hematologic malignancies, including acute myeloid leukemia (AML), multiple myeloma (MM), and lymphomas. Therefore, targeting MCL-1 has emerged as a promising therapeutic strategy for the treatment of these diseases.
B. Strategies for MCL-1 inhibition:
Small-molecule inhibitors:
Small molecule inhibitors, including S63845 and AMG 176, bind to the BH3 binding groove of MCL-1, leading to its degradation and induction of apoptosis in cancer cells. Despite showing promising preclinical efficacy, the development of small molecule inhibitors has been limited by challenges related to specificity and pharmacokinetics.
BH3-mimetics:
BH3-mimetics, on the other hand, target the BH3 domain of pro-survival BCL-2 family members, including MCL-1, and disrupt the interaction between pro-survival and pro-apoptotic proteins, leading to induction of apoptosis in cancer cells. Several BH3-mimetics that specifically target MCL-1, such as A-1210477 and AZD5991, have also demonstrated promising preclinical efficacy.
RNA interference:
RNA interference (RNAi) is a process in which small interfering RNA (siRNA) molecules can be used to silence gene expression. Several studies have shown that targeting MCL-1 using siRNA can induce apoptosis in cancer cells and enhance the efficacy of chemotherapy. However, the clinical application of RNAi-based therapies is still limited by delivery and off-target effects.
Other approaches:
Other approaches to target MCL-1 include the use of proteasome inhibitors, such as bortezomib, which can lead to the accumulation of pro-apoptotic proteins and induction of apoptosis. Additionally, targeting upstream regulators of MCL-1, such as c-Myc, NF-κB, and STAT3, has also shown promising results in preclinical studies.
C. Challenges in developing MCL-1 inhibitors:
Specificity:
One of the major challenges in developing MCL-1 inhibitors is achieving specificity. MCL-1 shares significant structural homology with other pro-survival BCL-2 family members, such as BCL-XL and BCL-2, which can lead to off-target effects of MCL-1 inhibitors. Therefore, it is essential to design inhibitors that can specifically target MCL-1 without affecting other BCL-2 family members.
Pharmacokinetics:
Another challenge in developing MCL-1 inhibitors is achieving optimal pharmacokinetics, including bioavailability, distribution, metabolism, and excretion. MCL-1 inhibitors must be able to penetrate cell membranes and reach their target in sufficient concentrations to induce apoptosis in cancer cells.
Resistance mechanisms:
Resistance to MCL-1 inhibition can result from various mechanisms, including upregulation of other pro-survival BCL-2 family members, mutations in the MCL-1 gene, and alterations in the protein degradation machinery. As such, combination therapies targeting multiple pro-survival proteins or addressing resistance mechanisms may be required to achieve optimal efficacy.
In conclusion, targeting MCL-1 shows promise as a therapeutic strategy for treating hematologic malignancies. Multiple approaches, such as small molecule inhibitors, BH3-mimetics, RNA interference, and others, are currently being explored in preclinical and clinical studies. However, challenges such as achieving specificity, optimal pharmacokinetics, and addressing resistance mechanisms need to be addressed. Despite these challenges, MCL-1 inhibition has the potential to improve outcomes for patients with these challenging diseases, and several MCL-1 inhibitors are in clinical trials. Developing effective MCL-1 inhibitors has significant potential to benefit patients with hematologic malignancies.
MCL-1 inhibitors
Clinical development of MCL-1 inhibitors for hematologic malignancies includes two main categories: small-molecule inhibitors and BH3 mimetics. Small-molecule inhibitors bind to the BH3-binding groove of MCL-1, preventing its interaction with pro-apoptotic BH3-only proteins. Promising small-molecule inhibitors in clinical development include AMG 176, S64315, AZD5991, and BI-9700. Preclinical studies have demonstrated their ability to induce apoptosis in MCL-1-dependent cells and sensitize cells to other therapies, such as chemotherapy and BCL-2 inhibitors. Early-phase clinical trials are currently underway to evaluate these inhibitors for the treatment of hematologic malignancies, including acute myeloid leukemia and multiple myeloma.
BH3 mimetics are small molecules that mimic the BH3 domain of pro-apoptotic BH3-only proteins and bind to the BH3-binding groove of anti-apoptotic proteins, including MCL-1. These inhibitors induce apoptosis by displacing pro-apoptotic proteins from anti-apoptotic proteins and promoting the formation of the apoptosome. Venetoclax is a BH3 mimetic that has been approved for the treatment of chronic lymphocytic leukemia and is being evaluated for the treatment of other hematologic malignancies, including acute myeloid leukemia and multiple myeloma. Preclinical studies have shown that venetoclax can synergize with other therapies, including MCL-1 inhibitors, to induce apoptosis in cancer cells.
Overall, the development of MCL-1 inhibitors represents a promising approach for the treatment of hematologic malignancies, particularly those that are resistant to current therapies. Early-phase clinical trials are ongoing to evaluate the safety and efficacy of these inhibitors, and further studies are needed to determine their optimal use in combination with other therapies.
Table 1 Selective MCL-1 inhibitors evaluated in preclinical drug combination studies
S63845:
S63845, a MCL-1 inhibitor, is currently in early-phase clinical trials for the treatment of hematologic malignancies and has demonstrated promising results in preclinical studies.[10] Developed by Servier Laboratories in collaboration with Vernalis plc, this small-molecule inhibitor selectively binds to the BH3-binding groove of MCL-1, leading to the displacement of pro-apoptotic BH3-only proteins and the induction of apoptosis in MCL-1-dependent cancer cells. Its efficacy has been demonstrated in various cancer types, such as acute myeloid leukemia, multiple myeloma, and lymphoma, while showing minimal effect on normal cells. Furthermore, S63845 has been shown to sensitize cancer cells to other therapies, including chemotherapy and BCL-2 inhibitors.
In preclinical studies, S63845 demonstrated significant antitumor activity in both in vitro and in vivo models of hematologic malignancies. In a study of acute myeloid leukemia, S63845 was shown to induce apoptosis in primary patient samples, including samples that were resistant to chemotherapy. In a study of multiple myeloma, S63845 was shown to synergize with the BCL-2 inhibitor venetoclax to induce apoptosis in cancer cells. These studies suggest that S63845 has the potential to improve outcomes for patients with these challenging diseases.
Early-phase clinical trials of S63845 are ongoing in patients with acute myeloid leukemia, multiple myeloma, and other hematologic malignancies. The primary objectives of these trials are to evaluate the safety, tolerability, and pharmacokinetics of S63845 and to determine the optimal dose and schedule for further clinical development. Secondary objectives include evaluating the pharmacodynamics of S63845, assessing its antitumor activity, and identifying potential biomarkers of response.
The initial findings from clinical trials investigating S63845 have been encouraging, with no observed dose-limiting toxicities and indications of antitumor activity in certain patients. A phase I trial examining S63845 in patients with relapsed or refractory acute myeloid leukemia reported an overall response rate of 26% and a median response duration of 4.9 months. Additionally, a phase Ib/II trial studying the combination of S63845 with venetoclax in patients with relapsed or refractory multiple myeloma demonstrated an overall response rate of 60% and a median progression-free survival of 11.8 months. These results are promising and suggest that S63845 may represent a valuable therapeutic option for individuals affected by these difficult-to-treat diseases.
While the early clinical trials of S63845 and other MCL-1 inhibitors have shown promising results, there are still challenges that need to be addressed. Achieving specificity is one of the primary challenges since MCL-1 is essential for normal cell survival. Additionally, optimizing the pharmacokinetics of these inhibitors is crucial to ensure sufficient exposure and distribution to cancer cells.
In conclusion, S63845 represents a promising approach in the development of MCL-1 inhibitors for hematologic malignancies that are resistant to current treatments. However, larger clinical trials are needed to evaluate the safety and efficacy of S63845, particularly when used in combination with other therapies. Addressing resistance mechanisms and optimizing the pharmacokinetics of MCL-1 inhibitors are essential for successful drug development in this area.
AMG176:
AMG176 is a small molecule inhibitor that selectively targets MCL-1, a Bcl-2 family protein critical for the survival of cancer cells. Overexpression of MCL-1 has been linked to resistance to chemotherapy and poor prognosis in many cancers. AMG176 binds to MCL-1, inducing apoptosis in cancer cells that rely on this protein for survival. Preclinical studies have demonstrated potent activity against a broad range of cancer cell lines, including AML, multiple myeloma, non-small cell lung cancer, and breast cancer[11]. Synergistic activity has been observed in AML models when used in combination with venetoclax or azacitidine, indicating the potential to enhance the efficacy of existing therapies.
AMG176, a selective small molecule inhibitor of MCL-1, demonstrated encouraging results in a phase 1 clinical trial with relapsed/refractory hematologic malignancies[13]. The study included 38 patients with AML, MDS, multiple myeloma, and DLBCL, who received AMG176 as a single agent or in combination with other therapies. The trial’s primary objective was to determine the safety and tolerability of AMG176, while the secondary objective was to evaluate its pharmacokinetics, pharmacodynamics, and preliminary efficacy.
The majority of patients had previously undergone multiple lines of therapy and were refractory or relapsed after their most recent treatment. However, AMG176 was generally well-tolerated with no dose-limiting toxicities or treatment-related deaths reported in the trial. The most common adverse events were fatigue, nausea, and anemia. These promising results suggest that AMG176 may have potential as a treatment option for hematologic malignancies that are resistant to current therapies. Further studies are needed to evaluate the safety and efficacy of AMG176 in larger clinical trials and in combination with other therapies.
AMG176 has shown promising activity in patients with AML and MDS, both hematologic malignancies characterized by abnormal blood cell production and differentiation. In patients with AML, the overall response rate (ORR) was 33%, with one patient achieving complete remission (CR) and three achieving complete remission with incomplete hematologic recovery (CRi). In patients with MDS, the ORR was 25%, with one achieving partial remission (PR) and two achieving hematologic improvement (HI). Furthermore, some patients had stable disease or minor responses, suggesting AMG176 may have activity in a broader range of patients.
Based on these results, ongoing clinical trials are evaluating AMG176 for various types of cancer. In a phase 1b trial, AMG176 is being combined with venetoclax for the treatment of relapsed/refractory AML or MDS, based on preclinical data showing synergistic activity between the two agents. In addition, AMG176 is being evaluated in combination with other therapies, such as chemotherapy and immune checkpoint inhibitors, in various solid tumors.
However, AMG176’s development has some challenges that need addressing, as with other MCL-1 inhibitors. One of the main challenges is achieving sufficient exposure and distribution to tumor cells since MCL-1 is present in both normal and cancerous cells. The development of resistance mechanisms, such as upregulation of other anti-apoptotic proteins, is another challenge that could limit the efficacy of MCL-1 inhibitors.
AZD5991:
AZD5991 is a novel MCL-1 inhibitor developed by AstraZeneca that is currently undergoing clinical trials for the treatment of various types of cancer. MCL-1 is a key anti-apoptotic protein that is overexpressed in many cancer cells, making it an attractive target for cancer therapy.[14]
AZD5991 is a small molecule inhibitor that binds to the BH3-binding groove of MCL-1 and displaces pro-apoptotic proteins, such as BAK and BIM, leading to induction of apoptosis in cancer cells. Unlike other MCL-1 inhibitors, AZD5991 is highly selective for MCL-1 and does not affect the function of other anti-apoptotic proteins, such as BCL-2 and BCL-XL.
Preclinical studies have demonstrated the efficacy of AZD5991 in inducing apoptosis in a variety of cancer cell lines, including acute myeloid leukemia, multiple myeloma, and non-small cell lung cancer. In vivo studies have also shown significant anti-tumor activity of AZD5991 in several mouse models of cancer, including xenograft models of acute myeloid leukemia and triple-negative breast cancer.
In a phase I clinical trial, AZD5991 exhibited favorable tolerability and demonstrated promising anti-tumor activity in patients with relapsed or refractory acute myeloid leukemia and other hematologic malignancies. Among the 38 patients who received escalating doses of AZD5991, the maximum tolerated dose was 600 mg twice weekly. Of the 31 patients evaluated, 5 (16%) achieved a partial response, and 7 (23%) had stable disease with a median duration of response of 3.2 months.
AZD5991 is also being investigated as a monotherapy and in combination with other agents for the treatment of solid tumors in an ongoing phase I/II clinical trial. The study aims to evaluate the safety and tolerability of AZD5991 and its efficacy in patients with advanced or metastatic solid tumors, including non-small cell lung cancer, breast cancer, and ovarian cancer. Preliminary results from the trial demonstrate that AZD5991 is well-tolerated and has displayed promising anti-tumor activity in patients with non-small cell lung cancer and other solid tumors.
One potential advantage of AZD5991 over other MCL-1 inhibitors is its high selectivity for MCL-1, which may reduce the risk of toxicity to normal cells. Additionally, the pharmacokinetic profile of AZD5991 allows for twice-weekly dosing, which may improve patient compliance and convenience.
Despite the promising results, there are also several challenges associated with the development of AZD5991 and other MCL-1 inhibitors. One of the main challenges is the development of resistance mechanisms, as cancer cells may upregulate other anti-apoptotic proteins or downregulate pro-apoptotic proteins in response to MCL-1 inhibition. Strategies to overcome resistance to MCL-1 inhibitors are currently being investigated, such as combining MCL-1 inhibitors with other targeted agents or chemotherapy.
In summary, AZD5991 is a highly selective MCL-1 inhibitor that has shown promising anti-tumor activity in preclinical and clinical studies. Ongoing clinical trials will provide further insight into the safety and efficacy of AZD5991 and its potential role in the treatment of cancer.
Other inhibitors:
In addition to S63845, AMG176, and AZD5991, there are several other MCL-1 inhibitors currently under development or in preclinical stages. Here are some examples:
A-1210477: A-1210477 is a selective MCL-1 inhibitor that targets the BH3-binding groove of MCL-1, leading to the displacement of pro-apoptotic proteins necessary for cell survival. It has demonstrated promising results in preclinical studies involving multiple myeloma, acute myeloid leukemia, and lymphoma models. However, a phase I clinical trial in patients with relapsed or refractory hematologic malignancies revealed limited clinical activity, although the drug was well-tolerated.[16]
UMI-77: UMI-77 is a small-molecule MCL-1 inhibitor developed by researchers at the University of Michigan. It binds to the BH3-binding groove of MCL-1 and induces apoptosis in cancer cells. Preclinical studies have demonstrated efficacy in a variety of cancer types, including leukemia, lymphoma, and solid tumors. However, there are currently no clinical trials evaluating UMI-77.[17]
MIM1: MIM1 is a synthetic peptidomimetic that selectively inhibits MCL-1. It works by targeting the BH3-binding groove of MCL-1 and inducing apoptosis in cancer cells. Preclinical studies have shown that MIM1 has potent anti-tumor activity in multiple myeloma, acute myeloid leukemia, and lymphoma models. However, there are currently no clinical trials evaluating MIM1.[18]
BI-907828: BI-907828 is a small-molecule MCL-1 inhibitor developed by Boehringer Ingelheim. It works by binding to the BH3-binding groove of MCL-1 and inducing apoptosis in cancer cells. Preclinical studies have demonstrated efficacy in a variety of cancer types, including leukemia, lymphoma, and solid tumors. However, there are currently no clinical trials evaluating BI-907828.[19]
Overall, the development of MCL-1 inhibitors has shown promise in treating a variety of hematologic malignancies and solid tumors. However, there are still several challenges that need to be addressed, such as achieving specificity and optimizing pharmacokinetics, as well as overcoming resistance mechanisms. Clinical trials will continue to evaluate the safety and efficacy of these inhibitors and will help determine their potential as a new class of cancer therapeutics.
The development of MCL-1 inhibitors has shown promise in treating hematologic malignancies and solid tumors. However, challenges still need to be addressed, including specificity, pharmacokinetics, and overcoming resistance mechanisms. Ongoing clinical trials will continue to evaluate the safety and efficacy of these inhibitors to determine their potential as a new class of cancer therapeutics.
Summary
MCL-1 is a critical anti-apoptotic protein highly expressed in various cancers, making it an attractive therapeutic target for cancer treatment. MCL-1 overexpression inhibits the apoptotic pathway, allowing cancer cells to evade death signals and continue to proliferate. Researchers have developed several approaches to target MCL-1, such as small molecule inhibitors, BH3-mimetics, RNA interference, and other methods. These strategies have shown promising results in preclinical studies and clinical trials and have the potential to overcome resistance to other anti-cancer therapies. However, targeting MCL-1 presents several challenges, including achieving specificity, optimizing pharmacokinetics, and overcoming resistance mechanisms. Active research is underway to address these issues and improve the effectiveness of MCL-1 inhibitors in cancer therapy.
Targeting MCL-1 is a promising strategy for cancer treatment, particularly in hematologic malignancies. However, further research and clinical trials are necessary to establish the safety and efficacy of MCL-1 inhibitors in treating various cancers. Ongoing studies may lead to the development of new and effective therapies for cancer patients. In conclusion, MCL-1 inhibition has the potential to offer significant benefits in cancer therapy, but overcoming the challenges in targeting MCL-1 will be crucial to realizing its full potential.
Reference:
- Hallek, M. (2019). Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. American journal of hematology, 94(11), 1266-1287.
- Warren, J. L., Harlan, L. C., Stevens, J., Little, R. F., & Abel, G. A. (2013). Multiple myeloma treatment transformed: a population-based study of changes in initial management approaches in the United States. Journal of clinical oncology, 31(16), 1984.
- Dufva, O., Pölönen, P., Brück, O., Keränen, M. A., Klievink, J., Mehtonen, J., … & Mustjoki, S. (2020). Immunogenomic landscape of hematological malignancies. Cancer Cell, 38(3), 380-399.
- Wei, A. H., Roberts, A. W., Spencer, A., Rosenberg, A. S., Siegel, D., Walter, R. B., … & Stein, A. (2020). Targeting MCL-1 in hematologic malignancies: Rationale and progress. Blood reviews, 44, 100672.
- Belmar, J., & Fesik, S. W. (2015). Small molecule Mcl-1 inhibitors for the treatment of cancer. Pharmacology & therapeutics, 145, 76-84.
- Touzeau, C., Maciag, P., Amiot, M., & Moreau, P. (2018). Targeting Bcl-2 for the treatment of multiple myeloma. Leukemia, 32(9), 1899-1907.
- Gong, J. N., Khong, T., Segal, D., Yao, Y., Riffkin, C. D., Garnier, J. M., … & Huang, D. C. (2016). Hierarchy for targeting prosurvival BCL2 family proteins in multiple myeloma: pivotal role of MCL1. Blood, The Journal of the American Society of Hematology, 128(14), 1834-1844.
- Vikström, I. B., Slomp, A., Carrington, E. M., Moesbergen, L. M., Chang, C., Kelly, G. L., … & Tarlinton, D. M. (2016). MCL-1 is required throughout B-cell development and its loss sensitizes specific B-cell subsets to inhibition of BCL-2 or BCL-XL. Cell death & disease, 7(8), e2345-e2345.
- Michels, J., Johnson, P. W., & Packham, G. (2005). Mcl-1. The international journal of biochemistry & cell biology, 37(2), 267-271.
- Kotschy, A., Szlavik, Z., Murray, J., Davidson, J., Maragno, A. L., Le Toumelin-Braizat, G., … & Geneste, O. (2016). The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature, 538(7626), 477-482.
- Caenepeel, S. R., Belmontes, B., Sun, J., Coxon, A., Moody, G., & Hughes, P. E. (2017). Preclinical evaluation of AMG 176, a novel, potent and selective Mcl-1 inhibitor with robust anti-tumor activity in Mcl-1 dependent cancer models. Cancer Research, 77(13_Supplement), 2027-2027.
- Caenepeel, S., Brown, S. P., Belmontes, B., Moody, G., Keegan, K. S., Chui, D., … & Hughes, P. E. (2018). AMG 176, a Selective MCL1 Inhibitor, Is Effective in Hematologic Cancer Models Alone and in Combination with Established TherapiesAMG 176 Alone and Combined in Hematologic Cancer Models. Cancer discovery, 8(12), 1582-1597.
- Spencer, A., Rosenberg, A. S., Jakubowiak, A., Raje, N., Chatterjee, M., Trudel, S., … & Roberts, A. (2019). A phase 1, first-in-human study of AMG 176, a selective MCL-1 inhibitor, in patients with relapsed or refractory multiple myeloma. Clinical Lymphoma, Myeloma and Leukemia, 19(10), e53-e54.
- Tron, A. E., Belmonte, M. A., Adam, A., Aquila, B. M., Boise, L. H., Chiarparin, E., … & Hird, A. W. (2018). Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nature communications, 9(1), 5341.
- Matulis, S. M., Gupta, V. A., Brown, I., Keats, J. J., Secrist, P., Cidado, J., … & Boise, L. H. (2018). Preclinical activity of novel MCL1 inhibitor AZD5991 in multiple myeloma. Blood, 132, 952.
- Wang, Q., & Hao, S. (2019). A‑1210477, a selective MCL‑1 inhibitor, overcomes ABT‑737 resistance in AML. Oncology letters, 18(5), 5481-5489.
- Abulwerdi, F., Liao, C., Liu, M., Azmi, A. S., Aboukameel, A., Mady, A. S., … & Nikolovska-Coleska, Z. (2014). A Novel Small-Molecule Inhibitor of Mcl-1 Blocks Pancreatic Cancer Growth In Vitro and In VivoMcl-1 Inhibitors as Potential Therapeutics for Pancreatic Cancer. Molecular cancer therapeutics, 13(3), 565-575.
- Paysant, H., Hedir, S., Justaud, F., Weiswald, L. B., El Dine, A. N., Soulieman, A., … & Poulain, L. (2021). Structural revision of the Mcl-1 inhibitor MIM1: synthesis and biological studies on ovarian cancer cells with evaluation of designed analogues. Organic & Biomolecular Chemistry, 19(41), 8968-8987.
- Rudolph, D., Reschke, M., Blake, S., Rinnenthal, J., Wernitznig, A., Weyer-Czernilofsky, U., … & Moll, J. (2018). BI 907828: A novel, potent MDM2 inhibitor that induces antitumor immunologic memory and acts synergistically with an anti-PD-1 antibody in syngeneic mouse models of cancer. Cancer Research, 78(13_Supplement), 4866-4866.