Mastering Metabolism: How mTOR Shapes Aging, Health, and Future Therapies
Abstract
The mechanistic Target of Rapamycin (mTOR) is a central regulator of cellular metabolism, integrating nutrient, energy, and hormonal signals to control growth, protein synthesis, lipid metabolism, and autophagy. As research advances, mTOR has emerged not only as a master controller of metabolic health but also as a promising therapeutic target for aging and age-related diseases. This blog post explores the distinct roles of mTORC1 and mTORC2, their impact on the metabolism of carbohydrates, lipids, proteins, and nucleotides, and how mTOR signaling intersects with longevity and disease progression. We also highlight cutting-edge therapeutic strategies—including selective mTORC1 inhibitors, dietary interventions, and personalized medicine approaches—that are reshaping our ability to modulate this critical pathway with precision. By understanding and harnessing the power of mTOR, we open new avenues for extending healthspan and treating chronic metabolic disorders.
Decoding mTOR: Why This Molecular Pathway Matters in Metabolic Health and Aging
In the ever-evolving field of metabolic research, few molecular pathways have garnered as much attention as mTOR—the mechanistic Target of Rapamycin. This protein kinase functions as a central regulatory hub that integrates signals from nutrients, hormones, and energy status to control cell growth, metabolism, and even lifespan. As scientists explore deeper into the roots of aging and chronic disease, mTOR stands out as a critical switchboard in both health and disease.
mTOR exists in two distinct protein complexes: mTORC1 and mTORC2. These complexes are evolutionarily conserved and serve different, yet interconnected roles in regulating key biological processes. mTORC1 is activated primarily by nutrient availability—such as amino acids, glucose, and cholesterol—while mTORC2 responds to hormonal cues like insulin and growth factors. Together, they modulate everything from protein synthesis and lipid metabolism to autophagy and cytoskeletal structure.
But why does mTOR matter beyond the cell? Because its impact spans the entire organism. Dysregulated mTOR signaling has been linked to obesity, type 2 diabetes, cancer, and neurodegeneration. Intriguingly, mTOR inhibition—particularly mTORC1—has been shown to extend lifespan in yeast, worms, flies, and mice. In mammals, rapamycin (an mTOR inhibitor) has delayed age-related decline in multiple tissues, from the brain to the heart.
This dual role—regulating cellular growth and contributing to systemic aging—has placed mTOR at the forefront of both metabolic and longevity research. Scientists now believe that by precisely modulating mTOR activity, we could develop targeted therapies to treat age-related diseases and promote healthier aging.
As research accelerates, mTOR isn’t just a molecular pathway—it’s becoming a therapeutic target with extraordinary potential.
mTORC1 vs. mTORC2 – Two Complexes, Two Roles
The mTOR pathway operates through two distinct but interrelated complexes: mTORC1 (mTOR Complex 1) and mTORC2 (mTOR Complex 2). While both share the catalytic mTOR kinase, they differ in their accessory proteins, activation mechanisms, and physiological roles—making them functionally unique players in metabolic regulation.
mTORC1 acts as the primary nutrient sensor. It is acutely sensitive to rapamycin and is activated by amino acids, glucose, cholesterol, and energy status. When conditions are favorable, mTORC1 is recruited to the lysosome, where it interacts with Rheb-GTP, triggering downstream signals that promote anabolic processes like protein and lipid synthesis, while suppressing catabolic processes like autophagy.
On the other hand, mTORC2 is activated by growth factors such as insulin, IGF-1, and leptin. Unlike mTORC1, it is largely rapamycin-insensitive in the short term. mTORC2 plays critical roles in cell survival, cytoskeletal organization, and metabolic homeostasis by phosphorylating AKT at Ser473 and other downstream targets.
The two complexes also engage in feedback regulation. mTORC2 can enhance mTORC1 signaling via AKT, while mTORC1 can inhibit mTORC2 through negative feedback mechanisms involving S6K1 and IRS proteins. This fine-tuned interplay ensures cellular growth is balanced with external cues.
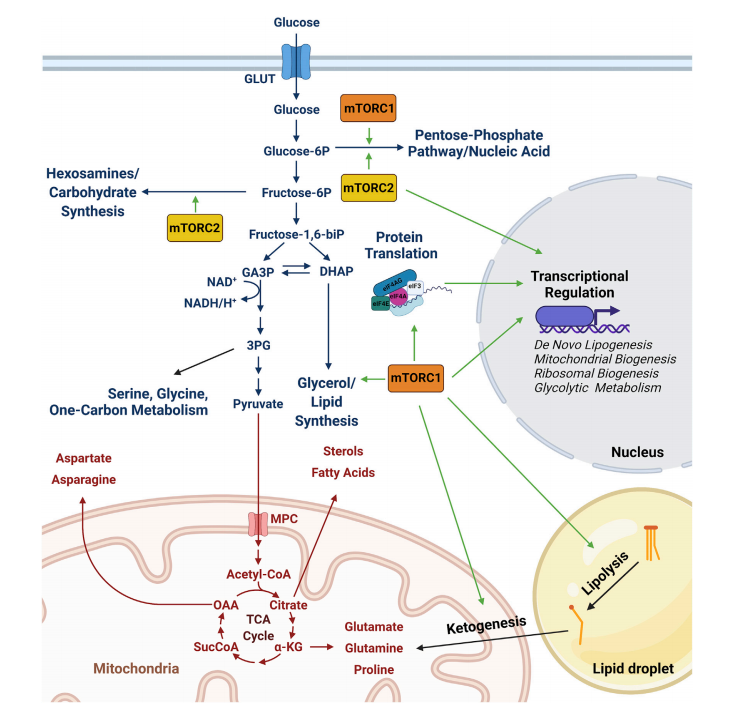
Figure 1. Control of cellular metabolism and other processes by mTORC1 and mTORC2
Understanding the differences between mTORC1 and mTORC2 is essential not just for molecular biology but for drug development. Targeting one complex without affecting the other could reduce side effects, particularly in therapies aimed at aging and metabolic disease.
Metabolism Under mTOR’s Command
One of mTOR’s most powerful roles lies in its ability to regulate the metabolism of the four essential macromolecular building blocks of the cell: carbohydrates, proteins, lipids, and nucleic acids. Through mTORC1 and mTORC2, this pathway dictates whether cells grow, store energy, or enter a catabolic state.
Carbohydrate metabolism is influenced by mTORC1’s sensitivity to glucose and the glycolytic intermediate DHAP. It modulates glucose uptake and glycolysis indirectly via AMPK and the TSC complex. mTORC2 plays a more direct role in insulin signaling and glucose homeostasis, making it critical for insulin sensitivity in tissues like liver and muscle.
Protein synthesis is tightly controlled by mTORC1, which phosphorylates S6K1 and 4E-BP1 to initiate translation and ribosome biogenesis. It also controls amino acid transporters and tRNA synthetases, linking nutrient availability directly to protein production.
Lipid metabolism is another domain of mTOR influence. mTORC1 promotes fatty acid synthesis through the SREBP1 transcription factor and inhibits lipid breakdown, favoring energy storage. Meanwhile, mTORC2 also contributes by modulating lipid gene expression in a tissue-specific manner, particularly in adipose tissue and the liver.
Nucleic acid metabolism is governed through mTORC1’s regulation of the pentose phosphate pathway (PPP), enhancing nucleotide synthesis and redox balance. It controls enzymes like G6PD and CAD, essential for purine and pyrimidine biosynthesis. mTORC2 complements this via AKT-mediated effects on non-oxidative PPP components.
Together, mTORC1 and mTORC2 act as metabolic conductors, harmonizing signals to balance growth and resource utilization. This makes them key players not only in normal physiology but also in pathological states like cancer, obesity, and diabetes.
Aging and Disease – mTOR at the Crossroads
As science uncovers the molecular underpinnings of aging, the mTOR pathway has emerged as a central node connecting metabolic regulation to age-related diseases. Its pivotal role in coordinating nutrient sensing, protein synthesis, and cellular growth places mTOR—particularly the mTORC1 complex—at the heart of longevity research.
Over the past two decades, studies in model organisms such as yeast, C. elegans, fruit flies, and mice have consistently shown that reduced mTORC1 signaling leads to longer lifespans. For instance, treatment with rapamycin—an mTORC1 inhibitor—extends lifespan in mice even when administered late in life. This groundbreaking discovery has fueled growing interest in repurposing rapamycin and related compounds as anti-aging therapeutics.
Beyond its impact on longevity, mTOR influences the onset and progression of age-related diseases. Hyperactive mTOR signaling has been linked to conditions such as cancer, neurodegeneration, cardiovascular disease, and metabolic disorders. Inhibiting mTORC1 helps mitigate many of these conditions by promoting autophagy, reducing inflammation, improving mitochondrial function, and enhancing stem cell maintenance.
However, the relationship is not without complexity. Chronic rapamycin treatment can inadvertently inhibit mTORC2, resulting in insulin resistance and metabolic side effects. This has prompted the development of dosing strategies (e.g., intermittent treatment) and next-generation inhibitors that selectively target mTORC1 while sparing mTORC2.
Moreover, mTOR’s effects on aging are tissue-specific. For example, inhibition of mTORC1 in adipose tissue promotes metabolic health, while excessive inhibition in other tissues may impair regeneration or immune function. The challenge moving forward is to fine-tune mTOR modulation to maximize benefits without triggering unintended consequences.
In short, mTOR sits at the crossroads of aging and disease. Its inhibition holds immense promise for improving healthspan—but must be approached with precision to strike the right balance between repair, regeneration, and resilience.
Future Therapies – Targeting mTOR with Precision
The therapeutic potential of modulating the mTOR pathway is no longer a distant concept—it’s rapidly becoming a tangible reality. As research into mTOR’s role in aging and metabolic disease deepens, scientists are shifting their focus from broad inhibition to precision-targeted interventions that maximize benefits while minimizing side effects.
One of the key advances is the development of next-generation mTOR inhibitors that selectively block mTORC1 while sparing mTORC2. This distinction is critical: while mTORC1 inhibition confers metabolic and longevity benefits, mTORC2 inhibition is often associated with adverse effects like insulin resistance. Agents such as RapaLink-1 and bi-steric mTORC1 inhibitors are showing promise in preclinical studies, offering sustained mTORC1 suppression without disrupting mTORC2 functions.
In addition to pharmacological tools, dietary strategies are gaining traction as natural modulators of mTOR activity. Caloric restriction and specific amino acid limitations—such as methionine or branched-chain amino acids (BCAAs)—can effectively downregulate mTORC1 signaling. These approaches are particularly appealing because they mimic the benefits of mTOR inhibition without requiring drug intervention, and they may be easier to implement for long-term healthspan management.
Researchers are also exploring combination therapies, such as pairing mTOR inhibitors with insulin sensitizers or autophagy enhancers, to improve therapeutic outcomes. Moreover, clinical trials are underway to evaluate low-dose or intermittent rapamycin regimens in humans, as well as in companion animals like dogs, where the impact on lifespan and age-related decline is actively being monitored.
Looking ahead, personalized mTOR modulation is on the horizon. By integrating genetic, metabolic, and lifestyle data, future interventions may be tailored to an individual’s unique mTOR signaling profile—ushering in a new era of precision medicine for aging and chronic disease.
As our understanding of mTOR advances, so too does the potential to reshape how we approach aging—not merely as an inevitable decline, but as a condition that can be proactively managed and improved.
References
Simcox, J., & Lamming, D. W. (2022). The central moTOR of metabolism. Developmental Cell, 57(6), 691–700.
https://doi.org/10.1016/j.devcel.2022.02.024
Harrison, D. E., et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460(7253), 392–395.
https://doi.org/10.1038/nature08221
Saxton, R. A., & Sabatini, D. M. (2017). mTOR signaling in growth, metabolism, and disease. Cell, 168(6), 960–976.
https://doi.org/10.1016/j.cell.2017.02.004
Lamming, D. W. (2014). Diminished mTOR signaling: A common mode of action for endocrine longevity factors. SpringerPlus, 3, 735.
https://doi.org/10.1186/2193-1801-3-735
Laplante, M., & Sabatini, D. M. (2012). mTOR signaling in growth control and disease. Cell, 149(2), 274–293.
https://doi.org/10.1016/j.cell.2012.03.017
Sarbassov, D. D., et al. (2006). Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Molecular Cell, 22(2), 159–168.
https://doi.org/10.1016/j.molcel.2006.03.029
Ben-Sahra, I., et al. (2013). Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science, 339(6125), 1323–1328.
https://doi.org/10.1126/science.1228792
Düvel, K., et al. (2010). Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Molecular Cell, 39(2), 171–183.
https://doi.org/10.1016/j.molcel.2010.06.022
Porstmann, T., et al. (2008). PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene, 27(43), 5197–5208.
https://doi.org/10.1038/onc.2008.245
Kennedy, B. K., & Lamming, D. W. (2016). The mechanistic Target of Rapamycin: The grand conducTOR of metabolism and aging. Cell Metabolism, 23(6), 990–1003.