Mastering Growth: How the mTOR Pathway Controls Protein Synthesis and Shapes Human Health
Abstract
The mammalian target of rapamycin (mTOR) pathway plays a central role in regulating protein synthesis, cellular metabolism, and growth. This blog post explores how mTOR integrates signals from nutrients, hormones, and energy status to control the initiation and elongation phases of translation, as well as ribosome biogenesis. Through its two complexes, mTORC1 and mTORC2, the pathway orchestrates precise cellular responses to environmental conditions. Dysregulation of mTOR signaling is implicated in a wide range of diseases, including cancer, cardiac hypertrophy, and insulin resistance, making it a critical target for therapeutic development. By examining recent findings and mechanistic insights, we highlight the biological importance of mTOR and its growing relevance in translational medicine.
Decoding the mTOR Pathway: Why mTOR Is the Master Switch of Cell Growth
Cell growth, metabolism, and protein synthesis are intricately controlled processes that require seamless integration of nutrient signals, energy availability, and hormonal cues. At the heart of this regulation lies a pivotal kinase known as mTOR (mammalian target of rapamycin)—a master coordinator that ensures cells only grow and divide when conditions are favorable.
mTOR acts as a molecular “hub,” receiving input from growth factors like insulin, amino acids such as leucine, and cellular energy status. Once activated, it drives downstream processes that enable protein synthesis, ribosome biogenesis, and other anabolic functions. The pathway’s significance is underscored by its evolutionary conservation across species and its vital role in embryonic development—mice lacking mTOR die early in gestation due to developmental failure.
mTOR signaling occurs via two major complexes: mTORC1 and mTORC2. The first is rapamycin-sensitive and primarily involved in controlling protein synthesis and cell size. The second regulates cell survival and metabolism through its effects on Akt/PKB and is less sensitive to rapamycin. Through these complexes, mTOR orchestrates the initiation and elongation phases of mRNA translation, selectively boosting the synthesis of proteins required for growth and adaptation.
Importantly, dysregulation of mTOR has been implicated in a wide range of diseases. Hyperactivation of the mTOR pathway is linked to tumor growth, metabolic dysfunction, and cardiac hypertrophy. Conversely, targeted inhibition of mTOR using agents like rapamycin has shown clinical benefits in cancer therapy, organ transplantation, and even potential applications in age-related diseases.
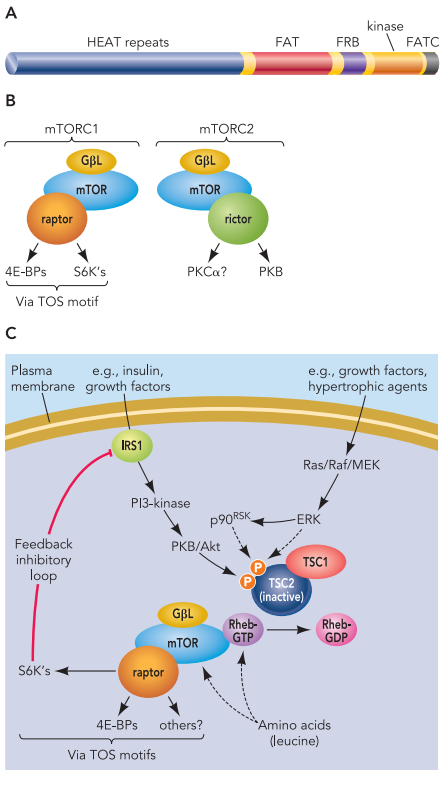
FIGURE 1. Features of mTOR
This blog series will explore how mTOR controls protein synthesis, the molecular players involved, and what this means for human health and disease. Understanding the mTOR pathway is not just about decoding a cellular mechanism—it’s about uncovering how life grows, adapts, and sometimes goes awry.
mTORC1 and mTORC2: Two Engines Driving Cellular Growth
The mTOR pathway exerts its powerful regulatory influence through two distinct protein complexes: mTORC1 and mTORC2. These complexes differ not only in their composition and sensitivity to drugs like rapamycin but also in their biological functions and downstream targets.
mTORC1 (mTOR Complex 1) is best known for its role in regulating protein synthesis, cell growth, and metabolism. It is acutely sensitive to rapamycin, which binds to the mTORC1 component FKBP12 and inhibits its activity. mTORC1 includes key proteins such as raptor, which helps recruit substrates like 4E-BP1 and S6K1 for phosphorylation (Wang & Proud, 2006). Once activated—often by signals such as insulin, IGF-1, amino acids (especially leucine), or increased energy status—mTORC1 enhances translation initiation and elongation, ribosome production, and nutrient uptake.
In contrast, mTORC2 (mTOR Complex 2), which includes rictor instead of raptor, is insensitive to acute rapamycin treatment and plays a vital role in regulating cytoskeletal dynamics and survival signaling pathways. mTORC2 activates Akt/PKB, a kinase involved in metabolism, proliferation, and inhibition of apoptosis.
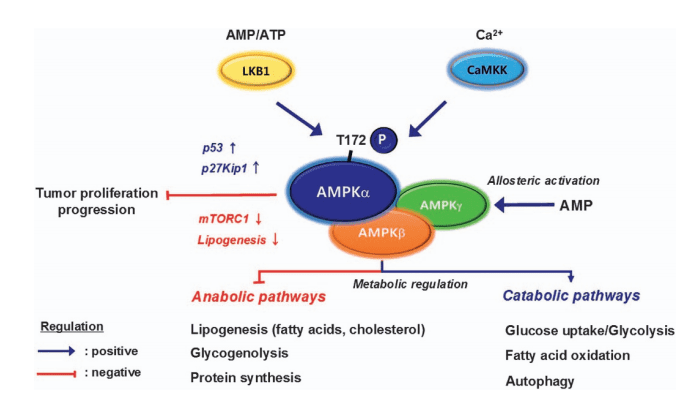
The upstream regulation of both complexes is sophisticated. Growth factors activate PI3K-Akt signaling, leading to the phosphorylation of TSC2 (tuberous sclerosis complex 2), which inhibits its GAP activity and allows Rheb, a small GTPase, to activate mTORC1). Additionally, MAPK/ERK signaling and energy-sensing pathways involving AMPK also converge on TSC2 to fine-tune mTORC1 activity.
This intricate control network enables cells to precisely match anabolic processes like protein synthesis to environmental inputs. Dysregulation at any point—such as mutations in TSC1/2 or chronic nutrient excess—can lead to diseases including cancer, obesity, and tuberous sclerosis.
Understanding how mTORC1 and mTORC2 function as twin engines of growth is essential for developing therapies that target metabolic and proliferative disorders at their root.
Initiation and Elongation: How mTOR Controls Protein Building
At the core of cellular growth lies the ability to make proteins efficiently—and the mTOR pathway is a master regulator of this process. Protein synthesis involves two critical steps: initiation, where ribosomes are recruited to mRNA, and elongation, where the peptide chain is extended. mTOR governs both phases by targeting specific translation factors, coordinating anabolic signals with biosynthetic activity.
During translation initiation, mTORC1 promotes the formation of the eIF4F complex, which includes eIF4E, eIF4G, and eIF4A. eIF4E binds the 5′-cap of mRNA, a necessary step for ribosome attachment. However, this function is tightly regulated by 4E-binding proteins (4E-BPs), which compete with eIF4G for eIF4E binding. When mTORC1 phosphorylates 4E-BPs, they release eIF4E, enabling translation to proceed.
mTORC1 also indirectly stimulates eIF4B, a helicase enhancer that helps unwind mRNA secondary structures. S6K1, activated downstream of mTORC1, phosphorylates eIF4B, facilitating the assembly of efficient initiation complexes.
In the elongation phase, mTOR controls the activity of eukaryotic elongation factor 2 (eEF2), which promotes ribosome translocation along mRNA. This is regulated through eEF2 kinase (eEF2K), an enzyme inhibited by mTORC1-dependent phosphorylation. By suppressing eEF2K, mTORC1 enhances eEF2 activity and accelerates elongation.
Moreover, under stress or low-energy conditions, AMPK activation overrides mTORC1, activating eEF2K and inhibiting elongation to conserve cellular resources. This regulatory balance ensures that protein production is aligned with nutrient availability.
In sum, mTOR doesn’t just switch protein synthesis on or off—it fine-tunes the machinery, ensuring efficiency under optimal conditions and restraint during stress. This dual control of initiation and elongation exemplifies mTOR’s central role in coupling growth signals to biosynthetic output.
Ribosome Biogenesis: mTOR’s Role in Building the Cellular Protein Factory
While mTOR’s regulation of translation initiation and elongation garners much attention, its influence extends deeper into the cell’s capacity to produce proteins—through the control of ribosome biogenesis. This process, which involves the synthesis of ribosomal proteins (r-proteins) and ribosomal RNA (rRNA), is critical for maintaining long-term protein synthesis and enabling sustained cell growth. mTOR coordinates both arms of this machinery, integrating nutrient and growth signals into the production of new ribosomes.
The mRNAs encoding ribosomal proteins are characterized by 5′-terminal oligopyrimidine tracts (5′-TOPs), which render them sensitive to translational control. Under nutrient-rich conditions or growth stimulation (e.g., insulin or serum), mTORC1 activation facilitates the shift of 5′-TOP mRNAs into polysomes, enhancing their translation. Interestingly, although S6K1 was once thought to mediate this effect, studies show that 5′-TOP mRNA translation can still occur in S6K1/2-deficient cells, implying the presence of alternative mTORC1-dependent mechanisms.
Beyond the cytoplasm, mTOR influences ribosome production at the nuclear level by regulating rRNA transcription, which occurs in the nucleolus via RNA polymerase I. mTOR activates UBF (Upstream Binding Factor) and TIF-IA, key transcription factors that drive rDNA transcription. When mTOR is inhibited—such as by rapamycin—TIF-IA is exported to the cytoplasm, disrupting rRNA synthesis and stalling ribosome assembly).
This dual control of r-protein and rRNA production underscores mTOR’s unique role as a central architect of the translational machinery. By synchronizing the expression of all ribosomal components, mTOR ensures that cells invest energy in growth only under favorable conditions. This coordination is especially relevant in tissues with high protein turnover, such as muscle, liver, and tumors.
Ultimately, mTOR’s regulation of ribosome biogenesis highlights how deeply embedded it is in the logic of growth: it doesn’t just press the gas on protein production—it also builds the engine.
Implications for Health and Disease: What Makes mTOR a Therapeutic Target
The mTOR pathway is more than just a regulator of cell growth—it is a central hub where metabolic signals, nutrient availability, and hormonal cues converge. Given its control over key biosynthetic processes, it is no surprise that dysregulation of mTOR signaling is a hallmark of numerous diseases, from cancer and metabolic disorders to cardiac hypertrophy and neurological conditions.
In cancer, mTOR signaling is often hyperactivated due to mutations in upstream regulators such as PI3K, PTEN, or TSC1/2. This unchecked activity promotes persistent protein synthesis, cell cycle progression, and survival—all contributing to tumorigenesis. In fact, many cancers rely on constitutive mTORC1 activity to maintain their anabolic state, making the pathway a high-priority target for therapeutic intervention.
Beyond oncology, mTOR plays a pivotal role in cardiovascular health, especially in the development of cardiac hypertrophy. In conditions of pressure overload or hormonal stimulation, mTORC1 drives increased protein synthesis in cardiomyocytes, leading to enlarged heart muscle cells. Although initially adaptive, this hypertrophic growth can progress to heart failure. Remarkably, animal studies show that treatment with rapamycin not only halts but can reverse established hypertrophy, demonstrating the therapeutic promise of mTOR inhibition in heart disease.
In metabolic disorders such as type 2 diabetes and obesity, mTOR’s role becomes more complex. While it supports insulin signaling, chronic nutrient excess and hyperactive mTORC1 can trigger negative feedback through S6K1-mediated phosphorylation of IRS1, contributing to insulin resistance—a key feature of metabolic syndrome.
Given this broad impact, targeting the mTOR pathway presents both opportunities and challenges. While rapalogs like rapamycin have opened the door to mTOR-based therapies, more selective and context-sensitive approaches are needed to exploit its full potential without unwanted side effects.
References
Wang, X., & Proud, C. G. (2006). The mTOR pathway in the control of protein synthesis. Physiology, 21, 362–369.
https://doi.org/10.1152/physiol.00024.2006
Browne, G. J., & Proud, C. G. (2004). A novel mTOR-regulated phosphorylation site in elongation factor 2 kinase modulates its activity. Molecular and Cellular Biology, 24(7), 2986–2997.
https://doi.org/10.1128/MCB.24.7.2986-2997.2004
Fattori, R., & Piva, T. (2003). Drug-eluting stents in vascular intervention. The Lancet, 361(9355), 247–249.
https://doi.org/10.1016/S0140-6736(03)12262-7
Gangloff, Y. G., Mueller, M., Dann, S. G., Svoboda, P., Sticker, M., Spetz, J. F., … & Kozma, S. C. (2004). Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Molecular and Cellular Biology, 24(21), 9508–9516.
https://doi.org/10.1128/MCB.24.21.9508-9516.2004
Gingras, A. C., Raught, B., & Sonenberg, N. (1999). eIF4 initiation factors: Effectors of mRNA recruitment to ribosomes. Annual Review of Biochemistry, 68, 913–963.
https://doi.org/10.1146/annurev.biochem.68.1.913
Gingras, A. C., Raught, B., Gygi, S. P., Niedzwieka, A., Miron, M., Burley, S. K., … & Sonenberg, N. (2001). Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes & Development, 15(22), 2852–2864.
https://doi.org/10.1101/gad.912401
Inoki, K., Zhu, T., & Guan, K. L. (2003). TSC2 mediates cellular energy response to control cell growth and survival. Cell, 115(5), 577–590.
https://doi.org/10.1016/S0092-8674(03)00929-2
Long, X., Lin, Y., Ortiz-Vega, S., Yonezawa, K., & Avruch, J. (2005). Rheb binds and regulates the mTOR kinase. Current Biology, 15(8), 702–713.
https://doi.org/10.1016/j.cub.2005.02.053
Ma, L., Chen, Z., Erdjument-Bromage, H., Tempst, P., & Pandolfi, P. P. (2005). Phosphorylation and functional inactivation of TSC2 by Erk: Implications for tuberous sclerosis and cancer pathogenesis. Cell, 121(2), 179–193.
https://doi.org/10.1016/j.cell.2005.02.031
Manning, B. D., & Cantley, L. C. (2003). Rheb fills a GAP between TSC and TOR. Trends in Biochemical Sciences, 28(11), 573–576.
https://doi.org/10.1016/j.tibs.2003.09.004
McMullen, J. R., Sherwood, M. C., Tarnavski, O., Zhang, L., Dorfman, A. L., Shioi, T., & Izumo, S. (2004). Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation, 109(24), 3050–3055.
https://doi.org/10.1161/01.CIR.0000130643.30103.5C
Raught, B., Peiretti, F., Gingras, A. C., Livingstone, M., Shahbazian, D., Mayeur, G. L., … & Sonenberg, N. (2004). Phosphorylation of eukaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO Journal, 23(9), 1761–1769.
https://doi.org/10.1038/sj.emboj.7600173
Sarbassov, D. D., Ali, S. M., & Sabatini, D. M. (2005). Growing roles for the mTOR pathway. Current Opinion in Cell Biology, 17(6), 596–603.
https://doi.org/10.1016/j.ceb.2005.09.008
Um, S. H., Frigerio, F., Watanabe, M., Picard, F., Joaquin, M., Sticker, M., … & Thomas, G. (2004). Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature, 431(7005), 200–205.
https://doi.org/10.1038/nature02866
Wang, X., Beugnet, A., Murakami, M., Yamanaka, S., & Proud, C. G. (2005). Distinct signaling events downstream of mTOR cooperate to mediate the effects of amino acids and insulin on 4E-BP1. Molecular and Cellular Biology, 25(7), 2558–2572.
https://doi.org/10.1128/MCB.25.7.2558-2572.2005
Wang, X., Li, W., Williams, M., Terada, N., Alessi, D. R., & Proud, C. G. (2001). Regulation of elongation factor 2 kinase by p90RSK1 and p70 S6 kinase. EMBO Journal, 20(16), 4370–4379.