Lumacaftor: Breakthroughs and Essential Compounds for Advanced Research
Abstract
Lumacaftor is a revolutionary drug that has garnered significant attention in the medical and pharmaceutical fields, primarily for its role in treating cystic fibrosis (CF). Cystic fibrosis is a genetic disorder characterized by the production of thick, sticky mucus that can clog the airways and lead to severe respiratory and digestive problems. The discovery and development of Lumacaftor have marked a pivotal moment in the treatment of this debilitating condition.
Keywords: Cystic Fibrosis, CFTR Modulators, Lumacaftor, Precision Medicine, Pharmaceutical Research
Lumacaftor functions as a corrector of the CF transmembrane conductance regulator (CFTR) protein, which is defective in individuals with cystic fibrosis. The CFTR protein is responsible for regulating the movement of chloride and sodium ions across epithelial cell membranes. In CF patients, mutations in the CFTR gene result in the misfolding and malfunctioning of this protein, leading to the characteristic symptoms of the disease. Lumacaftor works by stabilizing the defective CFTR protein, facilitating its proper trafficking to the cell surface, and partially restoring its function.
The importance of Lumacaftor in medical research extends beyond its immediate therapeutic effects. It represents a significant advancement in precision medicine, where treatments are tailored to address the underlying genetic causes of diseases. The development of Lumacaftor, often used in combination with ivacaftor, has provided a new lease on life for many CF patients, significantly improving lung function and reducing pulmonary exacerbations.
Recent studies have explored the potential repurposing of Lumacaftor for other medical conditions, including its use in targeting cancer stem cells. This highlights the drug’s versatility and the ongoing research efforts to fully understand its capabilities and broaden its therapeutic applications. Moreover, the clinical use of Lumacaftor has prompted extensive research into the long-term benefits and potential side effects, further emphasizing its importance in the pharmaceutical landscape.
The objective of this blog post is to provide an in-depth analysis of Lumacaftor, focusing on its mechanism of action, clinical applications, and the most recent research findings. In addition, we will explore the related chemical compounds that are integral to Lumacaftor research and development. These include impurities, building blocks, and isotope-labeled compounds, all of which are critical for ensuring quality control, facilitating drug synthesis, and advancing research methodologies.
Mechanism of Action and Clinical Applications
Mechanism of Action
Lumacaftor operates as a corrector of the cystic fibrosis transmembrane conductance regulator (CFTR) protein, which is defective in individuals with cystic fibrosis (CF). The CFTR protein plays a crucial role in regulating the movement of chloride and sodium ions across epithelial cell membranes, which is essential for maintaining the balance of salt and water on surfaces such as the lungs and pancreas. In CF patients, mutations in the CFTR gene lead to the production of misfolded CFTR proteins that are degraded before reaching the cell surface. Specifically, the F508del mutation, which is the most common CF mutation, causes the CFTR protein to misfold and subsequently get trapped in the endoplasmic reticulum, preventing it from reaching the cell surface where it is needed.
Lumacaftor addresses this defect by acting as a pharmacological chaperone. It binds to the misfolded CFTR protein, stabilizing its structure and facilitating its proper folding. This allows the CFTR protein to escape the degradation pathway, reach the cell surface, and function as a chloride channel. Although Lumacaftor does not fully restore normal CFTR function, it significantly improves chloride ion transport, thereby alleviating some of the symptoms associated with cystic fibrosis.
Clinical Applications
The primary clinical application of Lumacaftor is in the treatment of cystic fibrosis, particularly in patients who are homozygous for the F508del mutation in the CFTR gene. Lumacaftor is commonly used in combination with ivacaftor, another CFTR modulator, to enhance its therapeutic effects. This combination therapy, known as Orkambi, has been shown to improve lung function, reduce pulmonary exacerbations, and enhance the overall quality of life for cystic fibrosis patients.
Lumacaftor/ivacaftor therapy has been extensively studied and approved for use in both adult and pediatric patients. Clinical trials have demonstrated significant improvements in lung function (measured by the percent predicted forced expiratory volume in one second, or ppFEV1), reductions in sweat chloride levels (an indicator of CFTR function), and decreases in the rate of pulmonary exacerbations. These benefits are particularly pronounced in patients who initiate treatment at an early age, highlighting the importance of early intervention in managing cystic fibrosis.
Recent Advancements and Research Findings
Recent research has continued to explore and expand the potential applications of Lumacaftor. Studies have investigated its efficacy in other CFTR mutations beyond F508del, as well as its potential role in treating other diseases characterized by protein misfolding. For example, Lumacaftor has shown promise in preclinical studies for its ability to correct other CFTR mutations that cause misfolding and trafficking defects. This has important implications for expanding the therapeutic reach of Lumacaftor to a broader population of CF patients.
Additionally, the combination of Lumacaftor with other CFTR modulators is being explored to achieve synergistic effects and further enhance clinical outcomes. For instance, combining Lumacaftor with newer CFTR modulators like tezacaftor and elexacaftor has been shown to provide greater improvements in CFTR function and clinical outcomes compared to Lumacaftor/ivacaftor alone. These combination therapies represent a significant advancement in the treatment of cystic fibrosis, offering hope for improved management of this complex disease.
Ongoing clinical trials and real-world studies are crucial for understanding the long-term benefits and potential side effects of Lumacaftor, as well as identifying optimal dosing strategies and patient populations that could benefit the most from this therapy. The growing body of evidence supports the continued use and development of Lumacaftor-based therapies, highlighting their importance in the evolving landscape of cystic fibrosis treatment.
Current Research and Developments
Summary of Recent Research Studies on Lumacaftor
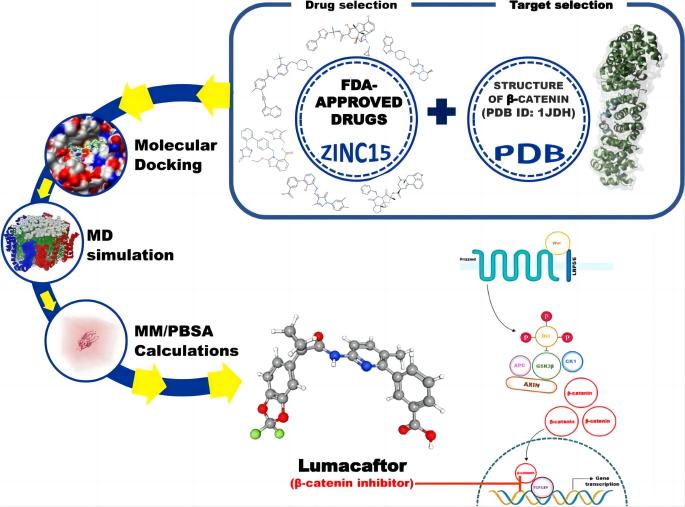
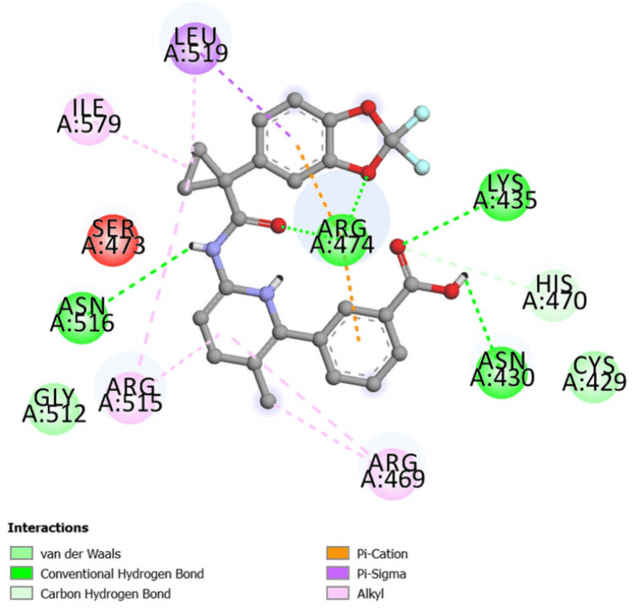
Lumacaftor has been the subject of extensive research in recent years, particularly concerning its efficacy and safety in the treatment of cystic fibrosis (CF). One of the significant studies published in the past two years explored Lumacaftor’s potential beyond its primary application in CF. A 2024 study by Pathak et al. examined the potential repurposing of Lumacaftor as a drug targeting breast cancer stem cells through in silico analysis. The study demonstrated that Lumacaftor, when bound to β-catenin, showed promising stability, suggesting its potential use in cancer therapy.
Another notable study conducted in 2023 evaluated the clinical experience of using Lumacaftor/Ivacaftor in children with CF in the Astrakhan region. The research highlighted the drug’s efficacy in improving clinical outcomes in young patients, despite some discontinuations due to adverse effects . This study underscores the importance of monitoring and managing side effects in pediatric populations.
Moreover, a 2024 study by Lindblad et al. assessed the clinical, economic, and societal impacts of CF and the benefits of Lumacaftor/Ivacaftor therapy. This comprehensive analysis using linked registry data confirmed the drug’s significant positive effects on patients’ quality of life and overall healthcare costs, reinforcing its value in managing CF.
Potential Future Applications in Other Medical Conditions
The promising results from recent studies have sparked interest in exploring Lumacaftor’s applications beyond cystic fibrosis. The in silico study on breast cancer stem cells is a prime example of how Lumacaftor might be repurposed to target other diseases characterized by protein misfolding and cellular dysfunction. The stabilization of β-catenin by Lumacaftor opens new avenues for cancer treatment, particularly in tumors where Wnt/β-catenin signaling is aberrant.
Additionally, ongoing research is investigating the use of Lumacaftor in other genetic disorders caused by protein misfolding. For example, preliminary studies suggest that Lumacaftor might be beneficial in certain types of muscular dystrophies and neurodegenerative diseases where protein misfolding and aggregation play a crucial role. These potential applications highlight the versatility of Lumacaftor and its broader therapeutic potential.
Challenges and Limitations in Current Research
Despite the promising advancements, several challenges and limitations remain in the research and application of Lumacaftor. One of the primary challenges is managing the side effects associated with Lumacaftor/Ivacaftor therapy. Adverse effects such as liver enzyme elevations and respiratory symptoms have led to discontinuation in some patients, necessitating careful patient selection and monitoring during treatment.
Another limitation is the variability in patient response to Lumacaftor/Ivacaftor therapy. Genetic differences among patients, such as variations in CFTR mutations and other modifier genes, can influence the efficacy of the treatment. This variability underscores the need for personalized medicine approaches to optimize therapy for individual patients.
Moreover, while in silico and preclinical studies provide valuable insights, there is a need for more clinical trials to validate the efficacy and safety of Lumacaftor in new therapeutic applications. Rigorous clinical testing is essential to translate these preliminary findings into viable treatments for other conditions.
In conclusion, while Lumacaftor has significantly advanced the treatment of cystic fibrosis, ongoing research is exploring its broader potential in medicine. Recent studies have highlighted its promise in cancer therapy and other genetic disorders, although challenges such as side effect management and patient variability remain. Continued research and clinical trials are crucial to fully realize the therapeutic potential of Lumacaftor and expand its applications in healthcare.
Provision of Related Chemicals
In the field of pharmaceutical research and development, the availability of high-quality chemical compounds is crucial for the success of drug discovery and development processes. Related chemicals, such as impurities, building blocks, and isotope-labeled compounds, play essential roles in ensuring the efficacy, safety, and advancement of new therapeutics. These compounds support various stages of drug development, from initial synthesis to clinical trials, providing critical insights into drug behavior and interactions.
Related Chemicals Available
Impurities
Impurities are unavoidable by-products of chemical synthesis processes. Their identification and quantification are vital for quality control and regulatory compliance. Understanding the presence and impact of impurities ensures that pharmaceutical products meet stringent safety and efficacy standards. Monitoring impurities helps in identifying potential toxicological risks and improving the overall purity of the drug substance, thereby safeguarding patient health.
Building Blocks
Building blocks are fundamental compounds used in the synthesis of more complex molecules. They are the essential components in the creation of pharmaceutical agents, enabling researchers to construct and modify drug candidates with desired properties. The availability of diverse building blocks accelerates the drug discovery process by allowing for the rapid generation and optimization of lead compounds. These building blocks facilitate the exploration of structure-activity relationships, leading to more effective and targeted therapeutics.
Isotope-Labeled Compounds
Isotope-labeled compounds are molecules in which specific atoms are replaced with their isotopic variants. These compounds are invaluable in research and diagnostic imaging due to their ability to trace biochemical pathways and monitor drug distribution and metabolism within the body. Isotope-labeled compounds are used in various applications, including pharmacokinetic studies, metabolic profiling, and the development of diagnostic imaging agents. Their precise tracking capabilities provide detailed insights into the pharmacological behavior of drug candidates.
Support for Ongoing Research and Development
The provision of related chemicals such as impurities, building blocks, and isotope-labeled compounds is fundamental to the ongoing research and development efforts in the pharmaceutical industry. These compounds enhance the understanding of drug mechanisms, improve quality control, and facilitate the creation of new and more effective medications. By supporting rigorous testing and innovation, these chemicals contribute to the advancement of medical science and the development of safer, more effective therapeutic options.
Conclusion and Future Perspectives
Lumacaftor has made a profound impact on the treatment of cystic fibrosis (CF), particularly for patients with the F508del CFTR mutation. By correcting the misfolded CFTR protein, Lumacaftor significantly improves lung function and reduces disease symptoms, enhancing the quality of life for many patients. Its role in precision medicine exemplifies how targeted therapies can address the underlying genetic causes of diseases, offering tailored and effective treatment options.
The advancement of Lumacaftor research and development is supported by the availability of crucial chemical compounds, including impurities, building blocks, and isotope-labeled compounds. These related chemicals are essential for ensuring the quality, safety, and efficacy of pharmaceuticals. Impurities are monitored for quality control, building blocks facilitate the synthesis of complex molecules, and isotope-labeled compounds enable detailed pharmacokinetic and metabolic studies.
Looking ahead, the potential repurposing of Lumacaftor for other conditions, such as cancer, highlights its versatility and broad therapeutic potential. Ongoing research is exploring its efficacy in other genetic disorders and protein misfolding diseases. Future breakthroughs may involve new combination therapies that enhance its effectiveness and minimize side effects, further expanding its clinical applications.