Lixisenatide: Optimizing Postprandial Glucose Control in Type 2 Diabetes
Abstract
Lixisenatide is a GLP-1 receptor agonist that effectively manages type 2 diabetes mellitus (T2DM) by improving glycemic control through its dual action of stimulating insulin secretion and inhibiting glucagon secretion in a glucose-dependent manner. Clinical trials have consistently demonstrated that lixisenatide reduces important markers of T2DM progression, such as HbA1c levels, fasting plasma glucose levels, and body weight in T2DM patients. The drug is well-tolerated, with the most common side effect being gastrointestinal disturbances. Hypoglycemia is uncommon when lixisenatide is used alone; however, when combining it with other antidiabetic medications, caution is advised. Overall, lixisenatide is a valuable treatment option for individuals with T2DM, offering improved glycemic control and a favorable safety profile.
Introduction to Lixisenatide
Lixisenatide is a glucagon-like peptide-1 (GLP-1) receptor agonist that was developed to treat type 2 diabetes mellitus. It is a member of the GLP-1 receptor agonist class and mimics the actions of incretin hormones, namely GLP-1, which the body naturally produces in response to food intake. GLP-1 stimulates the pancreas to secrete insulin and reduces the secretion of glucagon, both of which are essential for maintaining balanced blood glucose levels. Additionally, Lixisenatide delays gastric emptying, which slows the absorption of glucose and helps control postprandial (after meal) blood sugar spikes, which is a crucial part of managing type 2 diabetes.
Lixisenatide has several advantages, one of which being its once-daily dosage schedule that promotes patient convenience and adherence. It can be taken either alone or in conjunction with other medications that lower blood sugar, especially in those who require improved postprandial glucose control. Because of its special ability to increase insulin secretion solely in response to rising blood sugar, it lowers the risk of hypoglycemia, a major side effect of diabetes treatment.
Lixisenatide has proven to be highly effective in reducing HbA1c levels in clinical trials, which is a crucial indicator of long-term blood sugar control. Because of its superior safety profile and capacity to reduce postprandial hyperglycemia, the medicine is a beneficial choice for individuals who need a therapeutic strategy in addition to oral drugs or basal insulin.
Despite its advantages, lixisenatide, like all GLP-1 receptor agonists, has the potential to have negative effects, such as nausea and vomiting, especially in the early stages of treatment. But these are usually fleeting and fade with repeated use.
Mechanism of Action
Lixisenatide functions as a glucagon-like peptide-1 (GLP-1) receptor agonist, which is an incretin hormone that is essential for maintaining glucose homeostasis. The incretin effect is often reduced in type 2 diabetics, resulting in insufficient insulin secretion in response to food consumption. Lixisenatide mimics this function by binding to pancreatic beta cell GLP-1 receptors to stimulate insulin release in a glucose-dependent manner. This means that insulin secretion is increased only when blood glucose levels are elevated, lowering the risk of hypoglycemia, a common side effect of diabetes medicines.
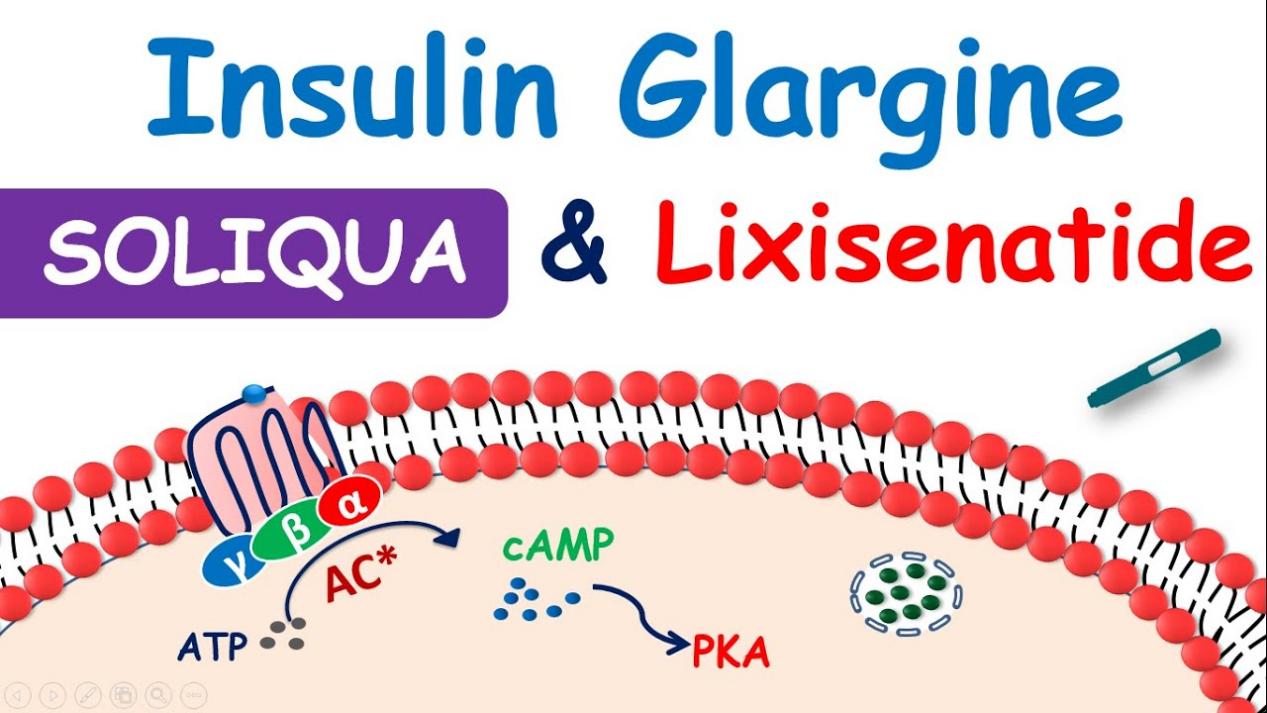
Fig.1 Lixisenatide:Mechanism of Action
Lixisenatide increases insulin secretion while inhibiting pancreatic alpha cells’ production of glucagon. The hormone glucagon causes the liver to produce more glucose, which in turn raises blood glucose levels. Lixisenatide contributes to the maintenance of ideal blood glucose levels by blocking glucagon, especially after meals. The delay in stomach emptying is a key component of its mechanism. Lixisenatide lowers the rate of glucose absorption into the bloodstream by delaying the passage of food from the stomach into the small intestine. This aids in reducing postprandial blood sugar increases, which are a significant problem in the management of type 2 diabetes.
Lixisenatide is an effective medication for glycemic management because of its three main actions: it increases insulin production, lowers glucagon levels, and slows stomach emptying. It has a clear benefit over other anti-diabetic drugs that could result in hypoglycemia because of its glucose-dependent effect on insulin secretion. Because of these advantages, lixisenatide is a useful medication for individuals with type 2 diabetes, both as a monotherapy and combination treatment.
Clinical Applications
For the treatment of type 2 diabetes, lixisenatide has received widespread approval. This is especially true for individuals who need improved postprandial glucose control. Its application as a GLP-1 receptor agonist has proven to be highly effective in enhancing glycemic control by a reduction in HbA1c levels, an important indicator of long-term blood sugar regulation. Patients who suffer with blood sugar increases after meals but whose fasting blood sugar levels are under control should pay particular attention to this. Lixisenatide is an essential treatment option for people with type 2 diabetes since clinical trials have repeatedly demonstrated its ability to successfully control postprandial hyperglycemia.
Apart from its potential to lower blood sugar, lixisenatide has also been linked to modest weight loss—a benefit that many obese type 2 diabetic patients find appealing. Its ability to slow stomach emptying and decrease appetite contribute to this effect. Lixisenatide can be used as monotherapy or in combination with other antidiabetic medications, like metformin, sulfonylureas, or basal insulin. This flexibility allows for customized treatment plans, particularly for patients whose current therapies are failing to achieve the desired HbA1c levels.
Due to its glucose-dependent insulin secretion, lixisenatide’s clinical use is especially advantageous for patients who require extra postprandial glucose management without running the risk of hypoglycemia. Because of its effectiveness and safety profile, lixisenatide has been recognized as a useful therapy choice for type 2 diabetes.
Combination Therapies
The combination of lixisenatide with other glucose-lowering treatments, especially basal insulin, has demonstrated remarkable promise. When combined with insulin, lixisenatide can be a useful tactic for patients who cannot attain ideal glycemic control by lowering postprandial glucose levels. With lixisenatide focusing on glucose spikes following meals and basal insulin controlling blood sugar levels during fasting, this combination effectively addresses both postprandial and fasting glucose. Clinical research has shown that adding lixisenatide to basal insulin reduces HbA1c significantly without significantly raising the risk of hypoglycemia or causing weight gain.
Furthermore, patients who have adequate fasting glucose control but high blood sugar after meals benefit most from the combination of lixisenatide and basal insulin. Lixisenatide’s capacity to reduce stomach emptying and increase glucose-dependent insulin secretion is utilized in this combo therapy. For patients who need more intensive care, the two drugs work in tandem to provide a more complete glycemic control strategy.
Lixisenatide is frequently used in conjunction with oral antidiabetic medications such sulfonylureas or metformin. Because of its adaptability, it can be included into a range of treatment plans, providing a versatile means of improving glycemic results. Patients can benefit from a multi-targeted approach to controlling blood glucose levels while reducing adverse effects, like the possibility of hypoglycemia, which is frequently linked to other medicines, by combining therapies.
Potential Side Effects
Lixisenatide has a well-established safety profile, similar to other GLP-1 receptor agonists, although it can also have specific side effects, particularly in the beginning of treatment. The gastrointestinal adverse effects—nausea, vomiting, and diarrhea—are the most often reported side effects. Usually mild to moderate in nature, these symptoms tend to go away as the body gets used to the medicine. The most common side effect is nausea, which usually starts during therapy and gets better with time. Lixisenatide is usually begun at a lower dose and increased gradually to reduce these side effects.
A headache or dizziness may also be experienced by certain patients in addition to gastrointestinal problems. When loxisenatide is administered either alone or in conjunction with metformin, hypoglycemia is not frequently experienced. However, using lixisenatide in conjunction with sulfonylureas or insulin raises the risk of hypoglycemia. Because of this, while starting lixisenatide, medical professionals frequently modify the dosages of other glucose-lowering drugs to lessen this risk.
Although rare, an allergic reaction is another possible side effect of lixisenatide. An allergic reaction can cause a rash, itching, and in more severe situations, breathing difficulties. Most lixisenatide side effects are mild and easily controlled, especially when doses are carefully adjusted. In general, lixisenatide is well tolerated. For the majority of patients, lixisenatide’s advantages in glycemic outcomes and postprandial glucose control exceed its drawbacks.
Ongoing Research and Advancements
The entire potential of lixisenatide is still being investigated, especially with regard to its cardiovascular advantages and possible uses in conjunction with other treatments. Interest has shifted to the cardiovascular safety of GLP-1 receptor agonists, with lixisenatide’s effects on the heart being examined in clinical trials. Lixisenatide’s safety profile in patients with cardiovascular disease was confirmed by the ELIXA trial, a significant evaluation of the drug in individuals with type 2 diabetes and a history of acute coronary syndrome. The trial showed that lixisenatide did not raise the risk of serious cardiovascular events.
Furthermore, research on lixisenatide’s possible advantages for managing weight is still underway. Patients using lixisenatide may have lost a little weight, according to certain studies. This is especially helpful for people with type 2 diabetes who frequently battle obesity. Lixisenatide’s weight-reducing benefits may be attributed to its ability to reduce hunger and slow stomach emptying, though larger trials are needed to fully investigate this.
Expanding the use of lixisenatide in conjunction with other antidiabetic treatments, such as more recent drugs, is another area of research interest. Combining treatments that target various facets of glucose regulation, such as SGLT2 inhibitors and basal insulin, enables medical professionals to deliver more individualized and efficient treatment choices. Combination therapy have the potential to improve patient outcomes, lower insulin dosages, and improve overall glycemic control.
Research on lixisenatide may go beyond managing diabetes in the future to address obesity and cardiovascular disease, for example. Lixisenatide may become more significant in customized treatment strategies as more data become available.
Conclusion
In the treatment of type 2 diabetes, lixisenatide has become a vital tool, especially for individuals who need to better regulate their postprandial glucose levels. Its mode of action targets multiple important components of efficient glycemic management, including inducing glucose-dependent insulin secretion, inhibiting glucagon release, and delaying stomach emptying. Consequently, lixisenatide has shown to be very successful in lowering postprandial hyperglycemia, lowering HbA1c levels, and assisting patients in maintaining more stable blood sugar levels throughout the day.
The adaptability of lixisenatide in treatment regimens is one of its main benefits. It offers versatility in managing diabetes because it can be used as monotherapy or in conjunction with other glucose-lowering drugs like metformin or basal insulin. This adaptability enables medical professionals to tailor care to the specific requirements of each patient, which is essential for maximizing therapeutic results.
Additionally, lixisenatide may help patients lose a small amount of weight, which is beneficial for a lot of type 2 diabetics who also happen to be overweight or obese. Even while gastrointestinal side effects including nausea and vomiting are frequent in the initial phases of treatment, they are usually temporary and can be controlled with dose modifications.
The potential of lixisenatide extends beyond conventional diabetes management, as evidenced by the current research into its cardiovascular effects and its function in combination therapy. Lixisenatide is a promising medication for long-term glycemic control due to its considerable efficacy and excellent safety profile. Lixisenatide may continue to be crucial in the management of type 2 diabetes and other metabolic disorders as the body of research expands, improving patient outcomes and quality of life.