Key Tumor Cell Targets for Antibody–Drug Conjugates (ADCs): From HER2 to TROP-2
Abstract
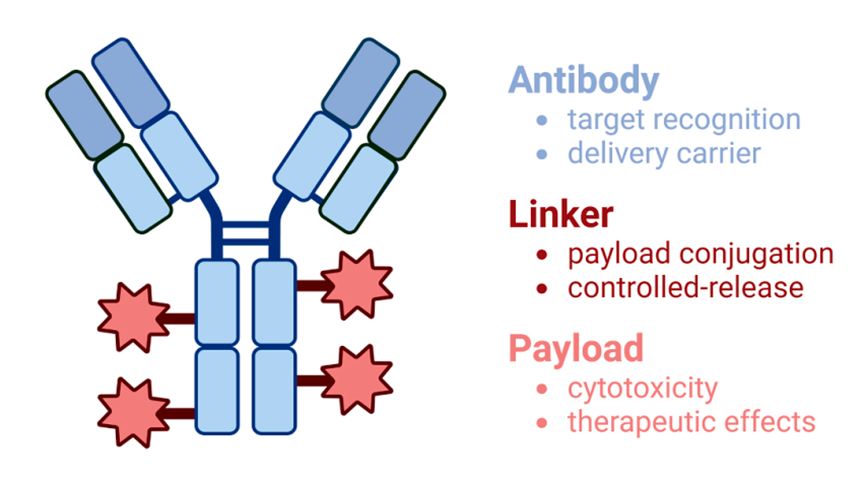
Antibody–drug conjugates (ADCs) are an emerging class of targeted cancer therapeutics that combine the specificity of monoclonal antibodies with the cytotoxic potency of chemotherapeutic agents. The choice of tumor cell surface antigen is critical to ADC efficacy and safety, influencing drug delivery, internalization, and off-target toxicity. This article reviews key tumor-associated antigens commonly targeted in both solid tumors and hematologic malignancies, including HER2, TROP-2, Nectin-4, and several CD family members. Target selection principles, representative ADCs, and their clinical applications are discussed, alongside emerging trends such as multi-target strategies and novel antigen discovery. Understanding the evolving landscape of ADC targets is essential for optimizing therapeutic index and guiding next-generation ADC development.
Introduction
Antibody–drug conjugates (ADCs) represent one of the most exciting innovations in modern oncology. By combining the tumor-selective targeting of monoclonal antibodies with the potent cytotoxicity of small-molecule payloads, ADCs aim to maximize anti-tumor activity while minimizing systemic toxicity.
At the heart of every ADC’s success lies a crucial factor: the target antigen. The choice of a tumor cell surface target determines not only where the ADC will bind, but also whether it will be internalized and release its cytotoxic payload effectively.
In this article, we explore the key tumor cell targets commonly used in approved and investigational ADCs, covering both solid tumors and hematologic malignancies, and look ahead to emerging trends in target discovery.
Principles of Target Selection for ADCs
Before diving into the list of targets, it’s important to understand the criteria scientists use when deciding on a suitable ADC antigen:
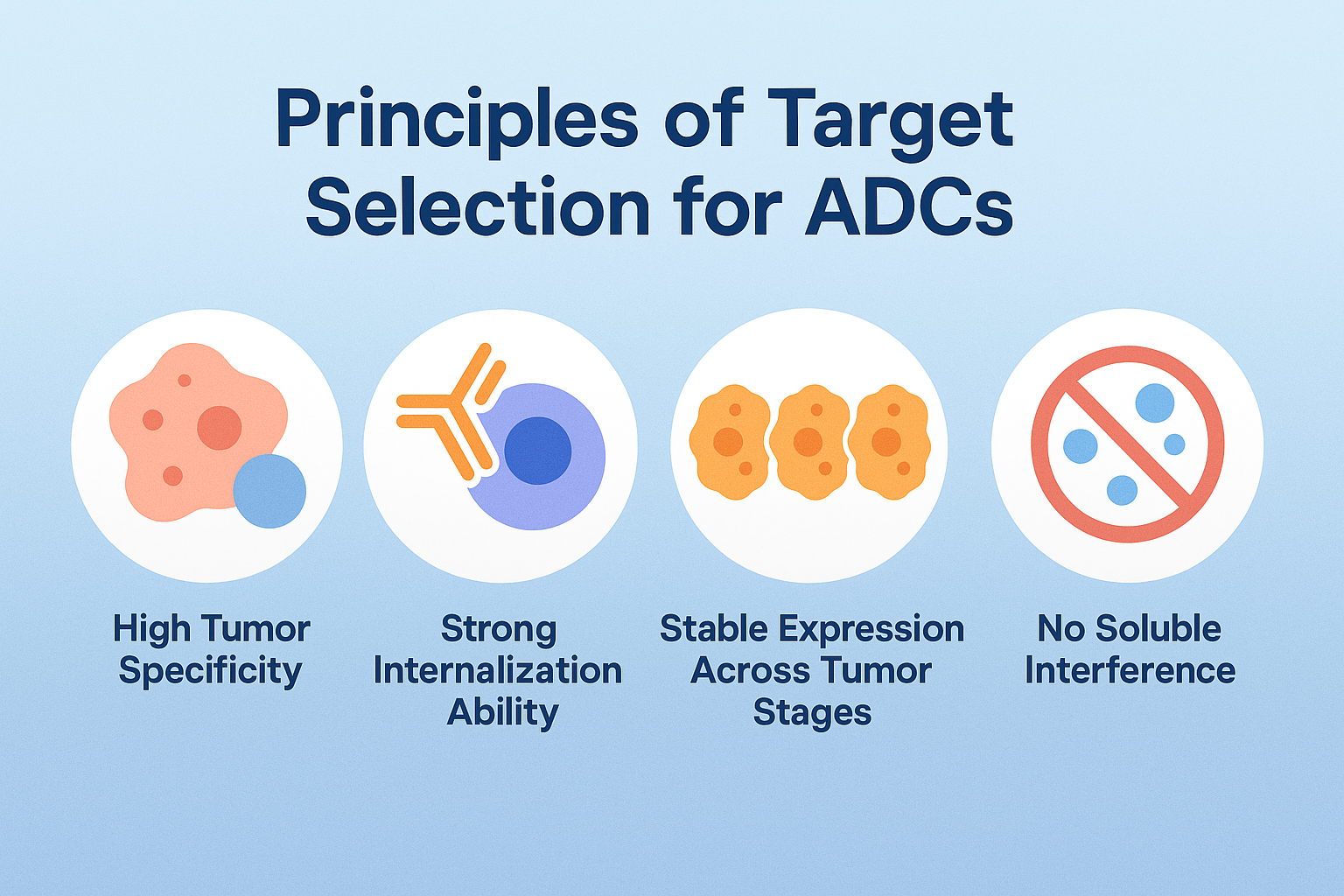
High Tumor Specificity
The target should be overexpressed on tumor cells and have minimal or no expression in normal tissues to reduce off-target toxicity.
Strong Internalization Ability
Once the ADC binds, the target–antibody complex should be efficiently internalized into the cell to enable payload release.
Stable Expression Across Tumor Stages
Ideal targets are expressed consistently across different patients, tumor stages, and metastatic sites.
No Soluble Interference
Targets should preferably lack a soluble form in circulation that could bind the ADC before it reaches tumor cells.
Key Targets in Solid Tumors
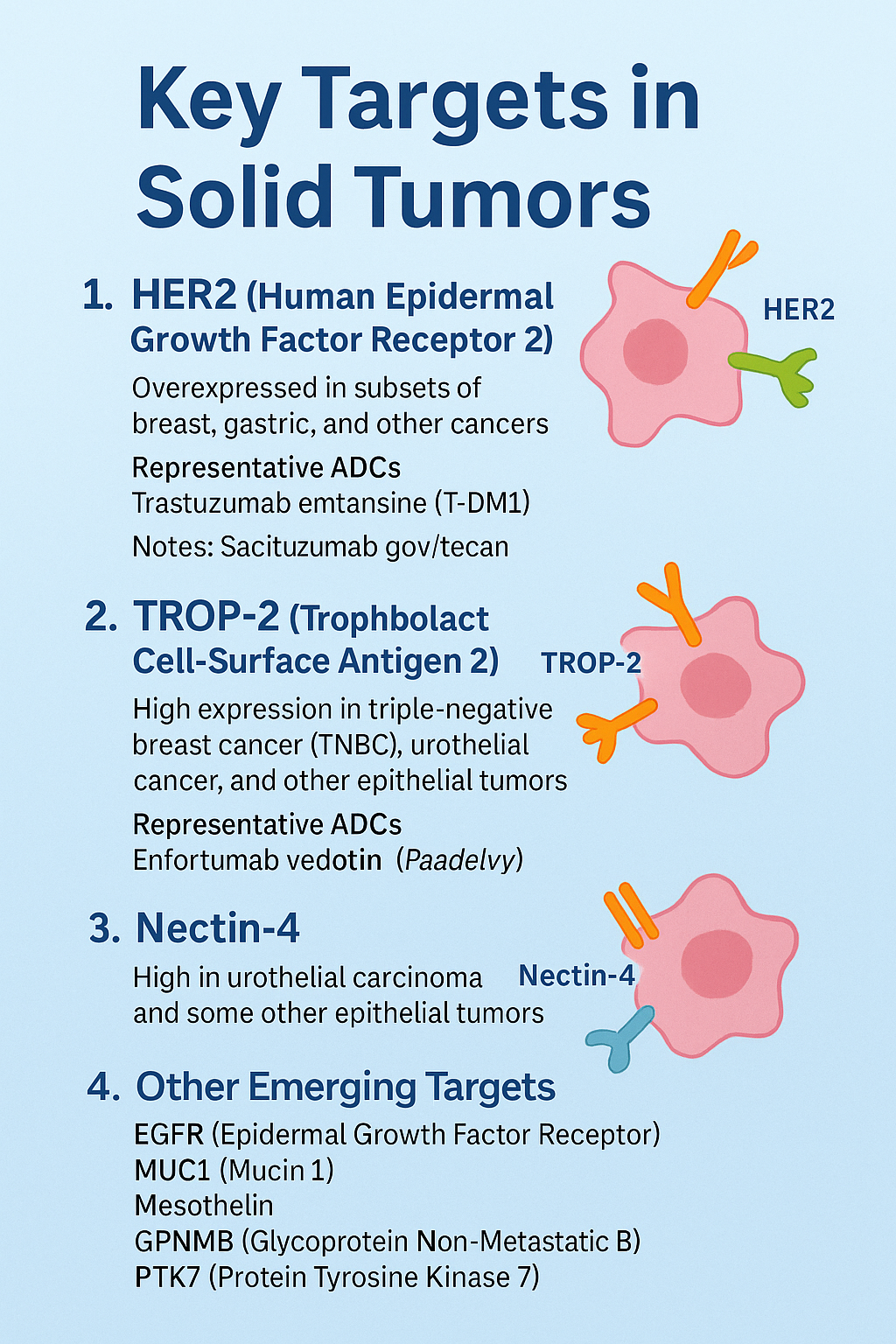
1. HER2 (Human Epidermal Growth Factor Receptor 2)
Expression profile: Overexpressed in subsets of breast, gastric, and other cancers.
Representative ADCs:
Trastuzumab emtansine (T-DM1)
Trastuzumab deruxtecan (Enhertu)
Notes: HER2 is a well-validated oncology target, with decades of clinical data from both monoclonal antibodies and ADCs.
2. TROP-2 (Trophoblast Cell-Surface Antigen 2)
Expression profile: High expression in triple-negative breast cancer (TNBC), urothelial cancer, and other epithelial tumors.
Representative ADCs:
Sacituzumab govitecan (Trodelvy)
Notes: TROP-2’s broad expression across solid tumors makes it a promising “pan-tumor” target.
3. Nectin-4
Expression profile: High in urothelial carcinoma and some other epithelial tumors.
Representative ADCs:
Enfortumab vedotin (Padcev)
Notes: Nectin-4 is part of the cell adhesion molecule family and shows favorable tumor selectivity.
4. Other Emerging Targets
EGFR (Epidermal Growth Factor Receptor)
MUC1 (Mucin 1)
Mesothelin
GPNMB (Glycoprotein Non-Metastatic B)
PTK7 (Protein Tyrosine Kinase7)
These targets are currently under active investigation in early-phase clinical trials.
Key Targets in Hematologic Malignancies
1. CD19
Indications: B-cell malignancies, including diffuse large B-cell lymphoma.
Representative ADC: Loncastuximab tesirine (Zynlonta)
2. CD22
Indications: B-cell acute lymphoblastic leukemia (B-ALL).
Representative ADC: Inotuzumab ozogamicin (Besponsa)
3. CD33
Indications: Acute myeloid leukemia (AML).
Representative ADC: Gemtuzumab ozogamicin (Mylotarg)
4. CD30
Indications: Hodgkin lymphoma, anaplastic large cell lymphoma.
Representative ADC: Brentuximab vedotin (Adcetris)
Target–Drug–Indication Snapshot
| Target | Representative ADC(s) | Cancer Type(s) |
|---|---|---|
| HER2 | T-DM1, Trastuzumab deruxtecan | Breast, gastric cancer |
| TROP-2 | Sacituzumab govitecan | TNBC, urothelial cancer |
| Nectin-4 | Enfortumab vedotin | Urothelial carcinoma |
| CD19 | Loncastuximab tesirine | B-cell lymphomas |
| CD22 | Inotuzumab ozogamicin | B-ALL |
| CD33 | Gemtuzumab ozogamicin | AML |
| CD30 | Brentuximab vedotin | Hodgkin lymphoma, ALCL |
Emerging Trends in ADC Target Discovery
Multi-Target Strategies
Bispecific antibodies combined with ADC payloads may overcome tumor heterogeneity and resistance.
Novel Antigens for Resistant Disease
Ongoing research focuses on targets upregulated in relapsed or treatment-resistant tumors, including those with low HER2 expression (“HER2-low” ADCs).
Advanced Screening Technologies
Single-cell sequencing, spatial transcriptomics, and high-throughput proteomics are accelerating the identification of new tumor-specific antigens.
Conclusion
Selecting the right tumor cell target is the foundation for any successful ADC therapy. The ideal target ensures that the cytotoxic payload is delivered precisely to tumor cells, sparing healthy tissues and improving therapeutic outcomes.
With more ADCs entering the clinic each year and a rapidly expanding list of validated antigens, the next generation of ADCs will likely feature more precise targeting, innovative payloads, and broader applicability across cancer types. For researchers and biotech companies, understanding the current and emerging target landscape is key to staying ahead in the ADC development race.
References
Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat Rev Drug Discov. 2017 May;16(5):315-337.
https://www.nature.com/articles/nrd.2016.268
Nagayama A, Vidula N, Ellisen LW, Bardia A. Novel antibody–drug conjugates for triple-negative breast cancer: spotlight on sacituzumab govitecan. Ther Adv Med Oncol. 2020;12:1758835920915980.
https://journals.sagepub.com/doi/full/10.1177/1758835920915980
Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384(12):1125–1135.
https://oak.ulsan.ac.kr/handle/2021.oak/8459
Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791.
https://www.nejm.org/doi/full/10.1056/NEJMoa1209124
Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380:741–751.
https://www.nejm.org/doi/full/10.1056/NEJMoa1814213
Zahavi D, Weiner LM. Monoclonal antibodies in cancer therapy. Antibodies. 2020;9(3):34.
https://www.mdpi.com/2073-4468/9/3/34
Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. 2012;30(7):631–637.
https://www.nature.com/articles/nbt.2289
Coats S, Williams M, Kebble B, et al. Antibody–drug conjugates: future directions in clinical and translational strategies to improve the therapeutic index. Clin Cancer Res. 2019;25(18):5441–5448.
Peters C, Brown S. Antibody–drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep.2015;35(4):e00225.
Lambert JM, Berkenblit A. Antibody–drug conjugates for cancer treatment. Annu Rev Med. 2018;69:191–207.
https://www.annualreviews.org/content/journals/10.1146/annurev-med-061516-121357