Keratan Sulfate, as a Therapeutic or Target Molecule
Abstract
Keratan sulfate (KS) is a glycosaminoglycan (GAG) type widely found in the extracellular matrices (ECM) of certain tissues, such as cornea, cartilage, and bone. KS is composed of a sulfated poly-Nacetyl lactosamine backbone. Its structure is formed by alternating 3-linkedβ-galactose (Gal) and 4-linked N-acetyl-β-glucosamine (GlcNAc) units displayed in disaccharide repeating building blocks within a polysaccharide chain[1].
Introduction
Keratan sulfate (KS) is a glycosaminoglycan (GAG) type widely found in the extracellular matrices (ECM) of certain tissues, such as cornea, cartilage, and bone. KS is composed of a sulfated poly-Nacetyl lactosamine backbone. Its structure is formed by alternating 3-linkedβ-galactose (Gal) and 4-linked N-acetyl-β-glucosamine (GlcNAc) units displayed in disaccharide repeating building blocks within a polysaccharide chain[1]. Although both units can be 6-O-sulfated, this modification occurs more often at the GlcNAc units[2] (Figure 1). KS is the only GAG type that does not bear an acidic residue such as glucuronic acid commonly seen in chondroitin sulfates, dermatan sulfate, hyaluronic acid, and heparin/heparan sulfate[3]; or iduronic acid commonly seen in dermatan sulfate and heparin/heparan sulfate. Instead of these acidic units, KS has the neutral sugar Gal, and this characteristic gives KS a less acidic potential in solution, once sulfation is the only acidic component of its structure.
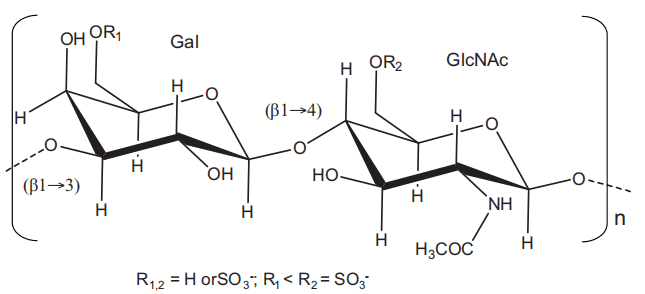
Figure 1 Structural representation of keratan sulfate. It is a polymer composed of disaccharide repetitions of alternating 3-linkedβ-galactopyranose (Gal) and 4-linked N-acetylglucosamine (GlcNAc). Sulfation occurs at 6-position of any monosaccharide but more often at the GlcNAc.
Fuction
KS, or keratan sulfate, plays a vital role in the biological functions of proteoglycans (PGs) found in the cornea, cartilage, and bone tissues. In the cornea, KSPGs are highly abundant and are responsible for maintaining appropriate hydration levels in the tissue. This hydration is crucial for ensuring the transparency of the cornea remains constant[4]. Maintaining transparency allows light beams to pass through and accurately converge on the retina, enabling proper vision.
If unexpected structural alterations occur in the KS chains of corneal PGs, it can lead to visual dysfunctions. This is evident in certain visual disorders such as macular corneal dystrophy and keratoconus[5]. Macular corneal dystrophy is characterized by defects or changes in the sulfation patterns of KS, while keratoconus is caused by dysfunction in KS chain formation.
However, both macular corneal dystrophy and keratoconus are caused by abnormalities in the organization of fibrils (collagens and PGs) in the cornea, leading to increased opacity of the tissue. To restore a healthier condition, eye drops containing KS as an active ingredient are often used. This is why KS is widely explored and utilized in the market as a key component of eye drops.
In addition to its role in the cornea, KS also plays a crucial role in cartilage. It helps maintain balanced hydration properties, particularly as a component of aggrecan, which effectively confers resistance to physical stress and loads on the tissue. Aggrecan is considered the largest molecular assembly in the body[6]. In bones, KS serves as a primary structural component in certain KSPGs that possess cell-binding properties.
Apart from its principal function in the cornea, KS also participates in developmental biology, cellular signaling, and migration, similar to other GAGs such as chondroitin, dermatan, and heparan sulfates. For example, while chondroitin sulfates promote neurite growth and guide neurite migration during neural development, KS-containing molecules act as barriers to neurite growth in vitro. KS and chondroitin sulfate appear to work together with opposing functions to maintain balanced mechanisms involved in neural development.
Applications in the biomedical field
As suppressor of cartilage damage
Dr. Ishiguro’s research group has published two papers in Biochemical and Biophysical Research Communications that explore the role of KS in suppressing cartilage damage. In their first paper from 2010, the researchers utilized a murine model of cartilage explants exposed to interleukin-1α (IL-1) to investigate the potential of KS through intraperitoneal administration in mitigating cartilage degradation[7]. They assessed cartilage fragility by measuring the release of IL-1α-induced aggrecan from the treated and untreated cartilage explants. The findings indicated that cartilage explants treated with KS exhibited lower levels of aggrecan release compared to those without KS administration.
In their subsequent paper from 2011, the authors further expanded their understanding of the potential therapeutic role of KS in cartilage damage using mice homozygous mutants for the enzyme N-acetylglucosamine 6-O-sulfotransferase (GlcNAc6ST) isoform 1 (GlcNAc6ST-1−/−) in comparison to wild-type mice(Figure 2)[8]. They employed a similar protocol to monitor the IL-1α-induced release of KS from the explants, as described in the previous paper. As expected, they observed higher levels of cartilage damage in the doubly allelic mutant mice. This finding supports the notion that naturally occurring KSPGs with normal 6-sulfation content play a vital role in slowing down the progression of cartilage damage.
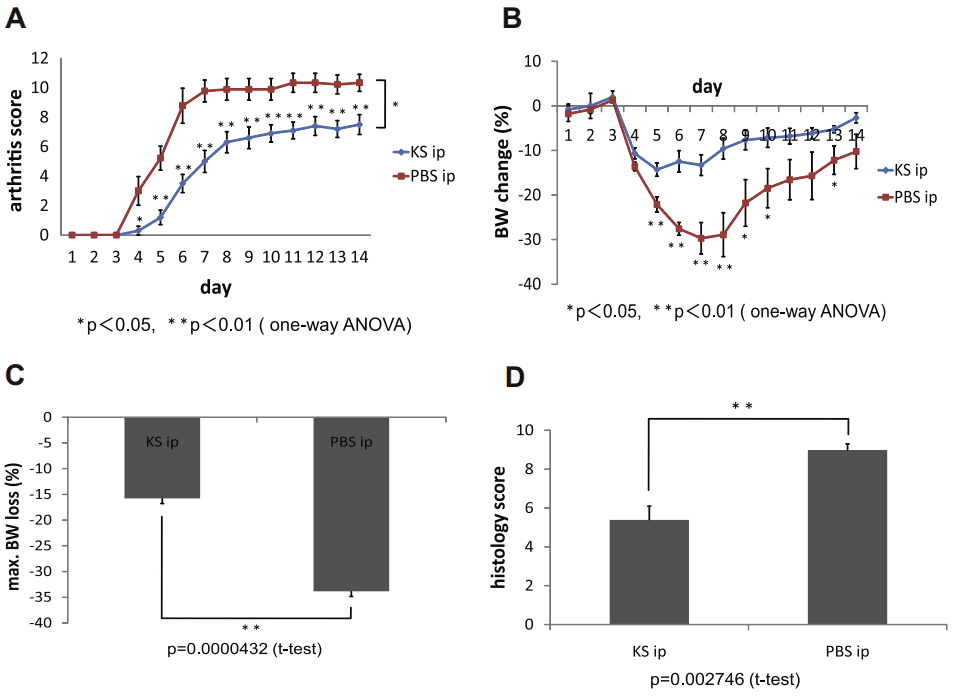
Figure 2 Suppression of antibody-induced arthritis by KS administration in GlcNAc6ST-1/ mice. (A) Time course for arthritis development in GlcNAc6ST-1/ mice treated with and without KS (n = 10/group). (B) Time course for changes in body weight (n = 10/group). (C) Maximum body weight loss (n = 10/group). (D) Histologic scores on day 7 (n = 6/group).[8]
Notably, the researchers demonstrated that the administration of KS formulations via intraperitoneal injection could suppress the damage, even in the mutant model. This highlights the potential therapeutic application of exogenous KS as a molecule for combating cartilage degradation.
As regulators of inflammation
In the papers above, the authors evaluated arthritis score, body weight loss, and histological conditions of joint cartilages in mice subjected to inflammatory conditions, with or without exogenous KS treatment. They found that KS administration significantly improved the condition of the inflamed animals, correlating with decreased levels of aggrecan release. This indicates that KS treatment inhibits aggrecan release from cartilage in vivo. The authors speculate that the amelioration of arthritis by KS is due to its chondroprotective mechanism. Similar results were observed in vivo mouse models of GlcNAc6ST-1-/-, where the IL-1-induced inflammation was worsened by the mutation but still improved by exogenous KS administration. Along with its beneficial effects on cartilage damage, KS is proposed as a novel therapeutic compound for inflammation.
Shirato and colleagues also demonstrated the potential of KS in attenuating inflammatory events in their publication in Biochemical and Biophysical Research Communications[9]. Instead of models of rheumatoid arthritis or osteoarthritis, this study focused on models of chronic obstructive pulmonary disease (COPD). COPD is characterized by inflammatory conditions driven by both immune cells and airway lung epithelial cells, resulting from bacterial and viral infections in the airways. KS has been identified as the major GAG type in airway secretions of COPD patients, synthesized by the airway surface epithelial cells under COPD conditions. The authors demonstrated that the KS disaccharide [Gal6S-(1-4)-GlcNAc6S], but not N-acetyl lactosamine or chondroitin 6-sulfate disaccharides, can suppress the production of interleukin-8 stimulated by flagellin, a Toll-like receptor 5 (TLR5) agonist found in normal human bronchial epithelial (NHBE) cells. This inhibition of flagellin-induced response impairs the phosphorylation of the epidermal growth factor receptor, disrupting the downstream signaling pathway via TLR5 in NHBE cells. These findings suggest that fully sulfated KS disaccharide may be used as a potent drug soon for the prevention and treatment of airway inflammatory conditions in COPD patients, especially in cases where bacterial infections drive or exacerbate the process.
In malignant cellular apoptotic process
Burkitt’s lymphoma, also referred to as Burkitt’s tumor or Burkitt lymphoma, is a type of cancer that affects the lymphatic system, specifically B lymphocytes. Nakayama and colleagues conducted a study to investigate the role of KSPGs (keratan sulfate proteoglycans) and their sulfation content in the radio-resistance of human Burkitt’s lymphoma cells during the apoptotic process[10].
In their experiments, the authors induced apoptosis in Burkitt’s lymphoma cell lines by subjecting them to X-ray radiation at a dose rate of approximately 2.4 Gy/min. To understand the impact of KSPG sulfation on this process, the researchers examined the involvement of sulfate donor transporters involved in KS biosynthesis, namely the 3′-phosphoadenosine 5′-phosphosulfate transporters (PAPST1 and PAPST2) and the GlcNAc6ST enzyme isoforms (CHST2, CHST6, and CHST7). The experiments were conducted on cell cultures exposed to varying levels of radiation. (Figure 3)
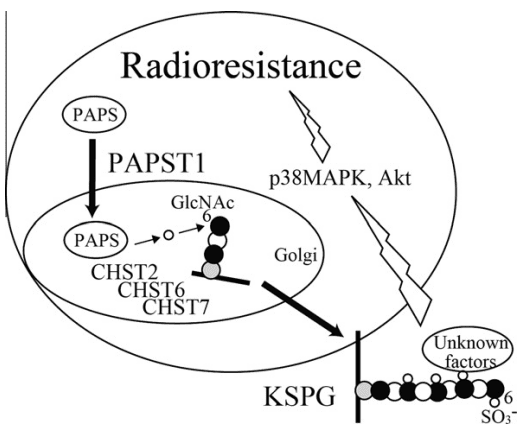
Figure 3 Scheme of the interaction of KS biosynthesis with radiation-induced apoptosis in Namalwa cells. PAPST1 supplies PAPS in the Golgi lumen. Sulfate is transferred to position 6 of GlcNAc residues in KS by all three GlcNAc6STs, resulting in the reduction of radiation-induced apoptosis through activation of p38 MAPK and Akt.[10]
The results of the study revealed that KSPGs, particularly the levels of GlcNAc 6-O-sulfation, play a role in reducing the apoptotic process induced by radiation in human Burkitt’s lymphoma cells. These findings provide insights for the future development of radiation-based anticancer therapies for Burkitt’s lymphoma. KSPGs can be considered as additional target molecules to enhance the effectiveness of radiation treatment. Combining radiation therapy with enzymes that degrade KS chains of KSPGs (known as keratinases) is likely to improve the quality, therapeutic efficacy, and outcomes of this cancer therapy.
As suppressors of amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a degenerative disease that affects motor neurons and has multiple causes. It is characterized by muscle spasticity, progressive weakness leading to muscle atrophy, and difficulties in speaking (dysarthria), swallowing (dysphagia), and breathing (dyspnea). Recent research by Hirano and colleagues has established a link between the absence of KS (keratan sulfate) and the increased development of ALS pathogenesis in its early stages [11].
In their study, the investigators compared ALS-induced mice models, including wild-type ALS-induced mice and GlcNAc6S-/- ALS-induced mice. The GlcNAc6S-/- mice were specifically modified to lack KS in their central nervous system tissues. Interestingly, the doubly allelic mutant mice exhibited more severe symptoms of ALS, demonstrating a direct correlation between KS sulfation content and the progression of the disease. In wild-type mice, KS expression was found exclusively in a subset of microglia, and its presence was reported only in the early stages of ALS. Unlike normal mice, which showed a temporary increase in microglia markers, this enhancement was significantly reduced in GlcNAc6S-/- mutant mice. KS expression by microglia was also observed in some human ALS cases. Overall, the study indicated that KS plays a crucial suppressive role in the early stages of ALS pathogenesis. While KS administration tests have not been conducted, the authors suggest the possibility of utilizing KS as a potential future drug candidate in therapeutic interventions against ALS.
Summary
Keratan sulfate (KS) is a glycosaminoglycan found in tissues such as the cornea, cartilage, and bone, playing important roles in maintaining tissue hydration and biological functions. It’s deficiency or structural changes can lead to visual disorders like macular corneal dystrophy and keratoconus. In cartilage, KS helps prevent degradation and is explored as a therapeutic molecule. It also exhibits anti-inflammatory properties, with potential applications in arthritis and chronic obstructive pulmonary disease (COPD) treatment. KS has been shown to suppress cartilage damage and inhibit aggrecan release in inflammatory conditions. In COPD, fully sulfated KS disaccharide has demonstrated the ability to suppress interleukin-8 production, making it a potential drug for airway inflammation in COPD patients. Moreover, KS is involved in the apoptotic process of Burkitt’s lymphoma cells, with studies showing that KSPGs and their sulfation content reduce radiation-induced apoptosis. Combining radiation therapy with enzymes degrading KS chains could improve Burkitt’s lymphoma treatment. Additionally, the absence of KS has been linked to increased development of amyotrophic lateral sclerosis (ALS) in its early stages, indicating a suppressive role in ALS pathogenesis. Although KS administration tests have not been conducted, it is considered a potential therapeutic candidate for ALS treatment. Overall, KS exhibits diverse functions and holds promise for therapeutic interventions in various diseases and conditions.
Reference
- Funderburgh, J. L. (2000). MINI REVIEW Keratan sulfate: structure, biosynthesis, and function. Glycobiology, 10(10), 951-958.
- Pomin, V. H., Piquet, A. A., Pereira, M. S., & Mourão, P. A. (2012). Residual keratan sulfate in chondroitin sulfate formulations for oral administration. Carbohydrate polymers, 90(2), 839-846.
- Pomin, V. H. (2014). NMR chemical shifts in structural biology of glycosaminoglycans. Analytical chemistry, 86(1), 65-94.
- Quantock, A. J., Young, R. D., & Akama, T. O. (2010). Structural and biochemical aspects of keratan sulphate in the cornea. Cellular and molecular life sciences, 67, 891-906.
- Pomin, V. H. (2015). Keratan sulfate: An up-to-date review. International Journal of Biological Macromolecules, 72, 282-289.
- Burg, M. A., & Cole, G. J. (1994). Claustrin, an antiadhesive neural keratan sulfate proteoglycan, is structurally related to MAP1B. Journal of neurobiology, 25(1), 1-22.
- Hayashi, M., Kadomatsu, K., & Ishiguro, N. (2010). Keratan sulfate suppresses cartilage damage and ameliorates inflammation in an experimental mice arthritis model. Biochemical and biophysical research communications, 401(3), 463-468.
- Hayashi, M., Kadomatsu, K., Kojima, T., & Ishiguro, N. (2011). Keratan sulfate and related murine glycosylation can suppress murine cartilage damage in vitro and in vivo. Biochemical and Biophysical Research Communications, 409(4), 732-737.
- Shirato, K., Gao, C., Ota, F., Angata, T., Shogomori, H., Ohtsubo, K., … & Taniguchi, N. (2013). Flagellin/Toll-like receptor 5 response was specifically attenuated by keratan sulfate disaccharide via decreased EGFR phosphorylation in normal human bronchial epithelial cells. Biochemical and Biophysical Research Communications, 435(3), 460-465.
- Hirano, K., Ohgomori, T., Kobayashi, K., Tanaka, F., Matsumoto, T., Natori, T., … & Kadomatsu, K. (2013). Ablation of keratan sulfate accelerates early phase pathogenesis of ALS. PLoS One, 8(6), e66969.