Hypoxia-Inducible Factor Pathway: Research and Therapeutic Application
Abstract
Small-molecule modulators of the HIF pathway have the potential to be effective therapeutic options for a range of diseases. These diseases include cancer, ischemic diseases, anemia, and metabolic diseases. However, there is a need for further research to address the challenges associated with developing these drugs and realize their full potential as therapeutics.
Introduction
Hypoxia-inducible factor (HIF) is a transcription factor that plays a critical role in oxygen homeostasis, metabolism, angiogenesis, and erythropoiesis. HIF is regulated by oxygen through the action of prolyl hydroxylases (PHDs) and factor inhibiting HIF (FIH), which modify HIF and target it for degradation or inhibition of its activity. Dysregulation of HIF has been implicated in a wide range of diseases, including cancer, ischemic diseases, anemia, and neurodegenerative diseases.[1-3]
Small-molecule modulators of the HIF pathway, such as PHD inhibitors, FIH inhibitors, and HIF-2α inhibitors, have been developed as potential therapeutics for these diseases. PHD inhibitors stabilize HIF by blocking its degradation, leading to increased HIF activity and downstream effects such as increased erythropoiesis and angiogenesis. FIH inhibitors increase HIF activity by blocking the inhibitory action of FIH, which regulates HIF activity under normoxic conditions. HIF-2α inhibitors specifically target HIF-2α, which is overexpressed in certain types of cancer and is associated with poor prognosis. HIF modulators have demonstrated great potential as therapeutic agents in both preclinical and clinical studies. Clinical trials for PHD inhibitors as treatments for anemia and ischemic diseases have shown promise, while HIF-2α inhibitors have shown activity in various cancers such as renal cell carcinoma. FIH inhibitors are still in early stages but have shown potential for treating metabolic diseases and cancer in preclinical studies. Despite the potential benefits, there are challenges that come with developing HIF modulators as therapeutics. These include optimizing drug efficacy and safety, identifying novel disease indications, developing combination therapies, and improving preclinical models for drug testing.
Mechanisms of HIF Regulation
The hypoxia-inducible factor (HIF) pathway is a critical regulatory pathway that plays a central role in the adaptation of cells to hypoxia, or low oxygen levels. HIF is a transcription factor that activates the expression of genes involved in a wide range of biological processes, including angiogenesis, erythropoiesis, glucose metabolism, and cell survival. The regulation of HIF is tightly controlled by a number of factors, including oxygen, prolyl hydroxylases (PHDs), and factor inhibiting HIF (FIH).
At normal oxygen levels, HIF is rapidly degraded through the ubiquitin-proteasome system. This process is regulated by a family of PHDs, which are oxygen-dependent enzymes that hydroxylate specific proline residues on the HIFα subunit. Hydroxylation of these proline residues creates a binding site for the von Hippel-Lindau (VHL) protein[11], which targets HIFα for ubiquitination and subsequent degradation(Figure 1).
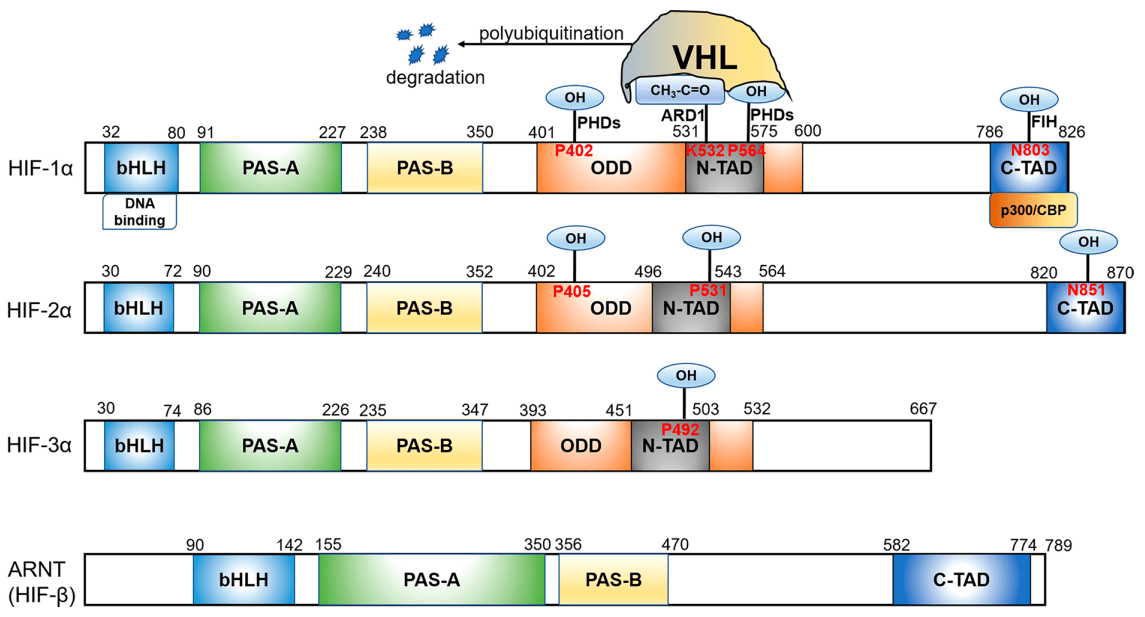
Figure 1 Functional domains of the HIF subunits[4]
Under hypoxic conditions, however, oxygen availability is limited, and the activity of PHDs is decreased. As a result, HIFα is stabilized and can translocate to the nucleus, where it dimerizes with the HIFβ subunit and binds to hypoxia response elements (HREs) in target gene promoters, leading to the transcriptional activation of HIF target genes.
In addition to PHDs, HIF activity is also regulated by FIH, which is another oxygen-dependent enzyme that hydroxylates an asparagine residue in the C-terminal transactivation domain of HIFα. This hydroxylation prevents the interaction of HIFα with the coactivator p300, resulting in the repression of HIF target genes. Thus, under normoxic conditions, FIH prevents the transcriptional activation of HIF target genes by inhibiting the interaction between HIFα and p300.[5,6]
Overall, the regulation of HIF by oxygen, PHDs, and FIH provides a critical mechanism for cells to respond to changes in oxygen availability and maintain homeostasis under hypoxic conditions. Dysfunction in this pathway can lead to a range of pathological conditions, including cancer, ischemic diseases, and anemia.
The HIF pathway has emerged as an attractive target for drug development, particularly in the context of cancer therapy. Inhibitors of PHDs have been developed that can stabilize HIF and activate the transcription of HIF target genes, leading to increased angiogenesis, glucose uptake, and cell survival. These effects can promote tumor growth and metastasis, making PHD inhibitors potential targets for cancer therapy.
HIF-2α inhibitors, which selectively block the transcriptional activity of a specific isoform of the HIFα subunit implicated in renal cell carcinoma, have been developed as potential cancer therapeutics, showing efficacy in reducing tumor growth and angiogenesis. The HIF pathway has also been targeted for the treatment of ischemic diseases, such as stroke and myocardial infarction, by promoting tissue repair and regeneration through the activation of HIF target genes involved in angiogenesis and erythropoiesis.
The development of HIF modulators represents a promising approach for the treatment of a wide range of diseases. Researchers are exploring new therapeutic avenues by manipulating the activity of HIF and its regulators, which hold potential in the treatment of cancer, ischemic diseases, and other pathological conditions.
Biological Functions of HIF
Hypoxia-inducible factor (HIF) is a transcription factor that plays a critical role in regulating oxygen homeostasis, metabolism, angiogenesis, and erythropoiesis. It is rapidly degraded under normal oxygen levels but accumulates and activates the transcription of target genes under hypoxic conditions, leading to cellular adaptation and survival.
HIF regulates oxygen homeostasis by promoting the expression of genes involved in glycolysis and reducing the expression of genes involved in oxidative phosphorylation under hypoxic environments. This shift in metabolism allows cells to generate energy under conditions of limited oxygen availability. HIF also regulates the expression of genes involved in the transport and storage of oxygen, including erythropoietin (EPO), which stimulates erythropoiesis and the production of red blood cells.
HIF plays a crucial role in angiogenesis by promoting the expression of genes involved in this process under hypoxic conditions, such as vascular endothelial growth factor (VEGF), which stimulates the growth of new blood vessels. This process is essential for the delivery of oxygen and nutrients to hypoxic tissues, facilitating tissue repair and regeneration.[7]
Hypoxia-inducible factor (HIF) is involved in various pathological conditions, including cancer and ischemic diseases, in addition to its role in oxygen homeostasis, metabolism, and angiogenesis. HIF promotes tumor growth and metastasis in cancer by stimulating angiogenesis, increasing glucose uptake, and promoting cell survival, as well as contributing to the epithelial-to-mesenchymal transition (EMT) that makes cancer cells more invasive and resistant to treatment.
Inhibition of HIF has been studied as a potential strategy for cancer therapy. Specifically, inhibitors of HIF-2α can selectively block its transcriptional activity, leading to decreased tumor growth and angiogenesis. Alternative approaches have targeted downstream effects of HIF activation, such as blocking the activity of vascular endothelial growth factor (VEGF), which is involved in tumor angiogenesis.
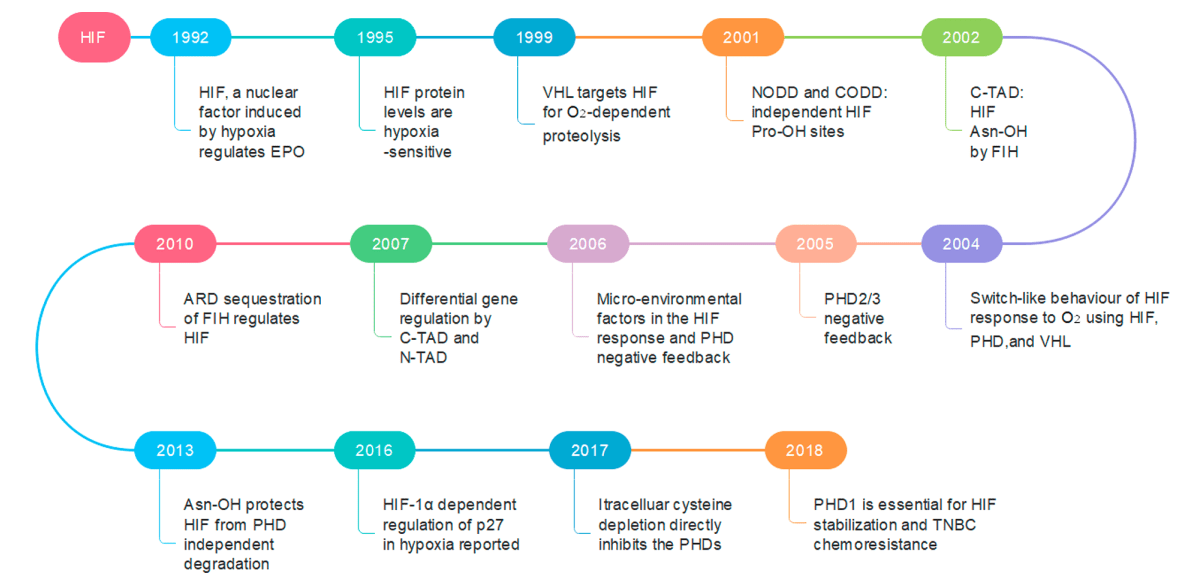
Figure 2 Profile of the main findings related to the critical components of the HIF pathway and the major modeled behaviors[4]
HIF has also been implicated in ischemic diseases such as stroke and myocardial infarction. Under such conditions, HIF activation can promote tissue repair and regeneration by stimulating angiogenesis and erythropoiesis, and promote cell survival under ischemia or reperfusion injury.
Pharmacological modulation of HIF activity has been explored as a potential therapy for ischemic diseases. For example, prolyl hydroxylase inhibitors (PHIs) have been developed that can stabilize HIF and activate the transcription of HIF target genes, leading to increased angiogenesis and erythropoiesis. PHIs have shown promise in preclinical models of ischemic diseases, but their clinical efficacy is still being evaluated.
Overall, HIF plays a critical role in the regulation of oxygen homeostasis, metabolism, angiogenesis, and erythropoiesis. Dysfunction in the HIF pathway can lead to a range of pathological conditions, including cancer and ischemic diseases. While targeting HIF represents a promising therapeutic strategy, further research is needed to better understand the complex regulation of this pathway and to develop safe and effective therapies that can selectively modulate HIF activity.
Small molecule modulators of HIF
The hypoxia-inducible factor (HIF) pathway plays a crucial role in cellular adaptation to low oxygen environments. As such, small molecule modulators of HIF have been developed as potential therapies for a range of diseases, including anemia, ischemic diseases, and cancer(Figure 3). In this response, we will provide an overview of three classes of small molecule modulators of HIF: prolyl hydroxylase inhibitors (PHIs), factor inhibiting HIF (FIH) inhibitors, and HIF-2α inhibitors.
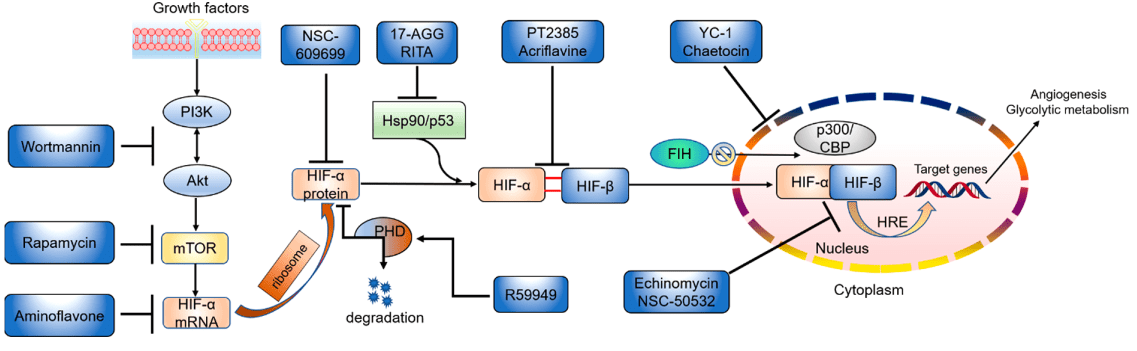
Figure 3 Small-molecule inhibitors of the HIF pathway targeting different sites[4]
PHIs are small molecule inhibitors of the prolyl hydroxylase (PH) enzymes, which are responsible for the oxygen-dependent degradation of HIF. PHIs bind to the active site of PH and prevent the hydroxylation of HIF, leading to its stabilization and activation[10]. This results in the upregulation of HIF target genes, such as EPO and VEGF, leading to increased erythropoiesis and angiogenesis, respectively.
Several PHIs have been developed, including FG-4592, vadadustat, and daprodustat, which are currently in clinical trials for the treatment of anemia associated with chronic kidney disease. These drugs have shown promising results in preclinical and clinical studies, demonstrating their potential as therapies for anemia. However, concerns have been raised regarding their safety, particularly their potential to stimulate tumor growth by increasing angiogenesis and cell survival.
FIH inhibitors are a relatively new class of small molecule modulators of HIF that target the FIH enzyme. FIH is responsible for the hydroxylation of an asparagine residue in HIF, which regulates its activity by inhibiting its interaction with the transcriptional co-activator p300/CBP[14]. FIH inhibitors prevent the hydroxylation of HIF and increase its activity by promoting its interaction with p300/CBP.
Several FIH inhibitors have been developed, including the compound IOX2, which has been shown to increase HIF activity in vitro and in vivo. IOX2 has been tested in preclinical models of ischemic diseases and has demonstrated protective effects by promoting tissue repair and regeneration. However, the clinical development of FIH inhibitors is still in the early stages, and further research is needed to determine their efficacy and safety in humans.
Small molecule inhibitors of HIF-2α have been developed as a strategy to target the critical role of this subunit in tumor growth and metastasis. By preventing the interaction of HIF-2α with its co-activators and inhibiting the transcription of HIF-2α target genes like VEGF and GLUT1, HIF-2α inhibitors can be effective in blocking the progression of cancer. Examples of such inhibitors include PT2385, PT2977, and MK-6482, which have demonstrated positive outcomes in preclinical and clinical studies for the treatment of renal cell carcinoma. However, there are concerns about the potential of these inhibitors to induce hypoxia and promote tumor metastasis by activating the HIF-1α pathway.
To summarize, PHIs, FIH inhibitors, and HIF-2α inhibitors are three classes of small molecule HIF modulators that offer potential as therapies for various diseases. PHIs have been effective in treating anemia associated with chronic kidney disease, but their safety is still a concern. FIH inhibitors and HIF-2α inhibitors are in early development stages, and more research is needed to establish their efficacy and safety in humans. Developing safe and effective therapies for HIF modulation requires a better understanding of the HIF pathway and its role in disease pathogenesis. Identifying biomarkers for monitoring treatment efficacy and safety is also important. Combination therapies targeting multiple HIF pathway components could provide a more effective approach to treating HIF-related diseases. While small molecule HIF modulators show promise for developing new therapies, further research is necessary to optimize their safety and efficacy.
Therapeutic Applications of HIF Modulators
Hypoxia-inducible factors (HIFs) are transcription factors that play a critical role in cellular adaptation to low oxygen conditions. HIFs regulate the expression of genes involved in several important biological processes, including angiogenesis, erythropoiesis, and glucose metabolism. Dysregulation of the HIF pathway has been implicated in a wide range of diseases, including cancer, ischemic diseases, and anemia. As a result, modulators of HIF have been investigated as potential therapies for these and other diseases.
Cancer is one of the most promising areas for the development of HIF modulators as therapeutics[8,9]. HIFs are known to play a critical role in tumor growth and metastasis by promoting angiogenesis, increasing glucose uptake, and promoting cell survival[12]. Inhibition of HIF has been explored as a potential strategy for cancer therapy, particularly inhibitors of HIF-2α that can selectively block the transcriptional activity of HIF-2α, leading to decreased tumor growth and angiogenesis. Other approaches have focused on targeting the downstream effects of HIF activation, such as blocking the activity of vascular endothelial growth factor (VEGF), which is involved in tumor angiogenesis. Several HIF-2α inhibitors have been developed, including PT2385, PT2977, and MK-6482, which have shown promising results in preclinical and clinical studies for the treatment of renal cell carcinoma. However, concerns have been raised regarding the potential for these drugs to induce hypoxia and promote tumor metastasis by activating the HIF-1α pathway. Combination therapies that target multiple components of the HIF pathway may provide a more effective approach to treating cancers that involve dysregulation of HIF.
HIF modulators have the potential to be used as therapeutics for ischemic diseases such as stroke and myocardial infarction. These diseases are characterized by reduced blood flow and oxygen supply to tissues, leading to tissue damage and cell death. HIFs have been found to play a critical role in the cellular response to ischemia by promoting angiogenesis and cell survival. Therefore, HIF activation has been explored as a potential strategy to promote tissue repair and regeneration following ischemic injury. However, the development of HIF modulators for the treatment of ischemic diseases has faced challenges due to the potential for HIF activation to also promote inflammation and fibrosis, which can worsen tissue damage. Thus, further research is required to determine the optimal timing and duration of HIF activation in the context of ischemic injury to minimize potential harmful effects.
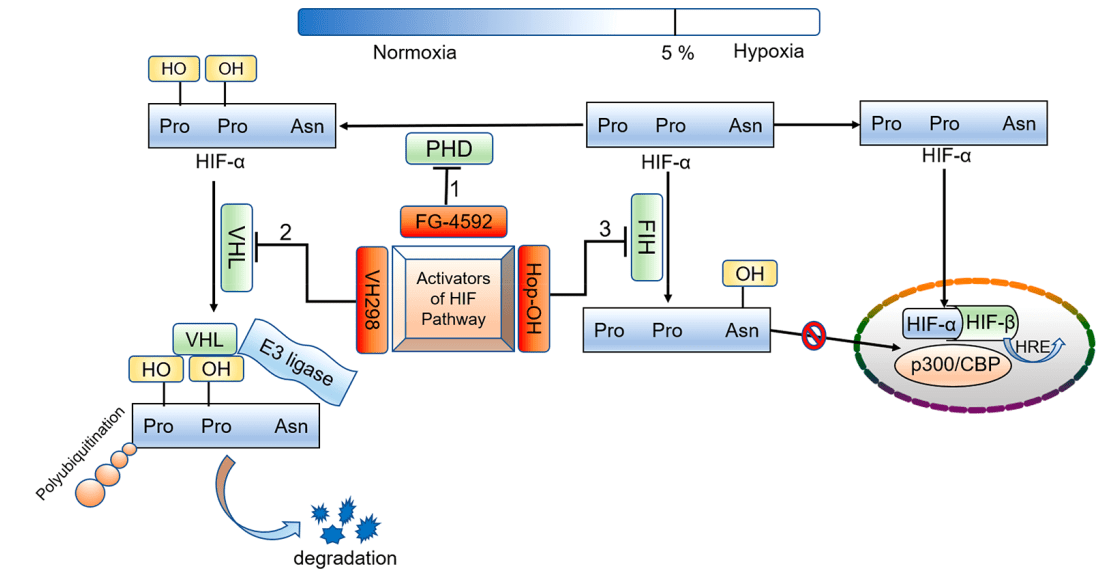
Figure 4 Oxygen-dependent regulation process of HIF-α and the potential strategies for activating the HIF pathway[4]
Anemia is a condition characterized by a decrease in the number of red blood cells or hemoglobin in the blood. HIF plays a critical role in the regulation of erythropoiesis, the process by which red blood cells are produced[13]. Hypoxia stimulates the production of erythropoietin (EPO), which in turn promotes the differentiation of erythroid progenitor cells into mature red blood cells. As a result, HIF activation has been explored as a potential strategy for the treatment of anemia. Prolyl hydroxylase inhibitors (PHIs), which prevent the degradation of HIF-α subunits, have been developed as a therapy for anemia associated with chronic kidney disease (CKD). PHIs have been shown to increase EPO production and improve hemoglobin levels in patients with CKD. However, concerns have been raised regarding the safety of PHIs, as chronic HIF activation may promote tumorigenesis and exacerbate other pathological conditions.
Small molecule modulators of PHD, such as roxadustat and vadadustat, have been developed as potential treatments for anemia associated with chronic kidney disease (CKD) and other conditions. Clinical studies have shown that these drugs are effective in increasing hemoglobin levels in patients with anemia and are well-tolerated. However, concerns have been raised regarding their safety, particularly with regards to their potential to increase the risk of cardiovascular events.
FIH inhibitors, such as IOX2, have also been shown to promote erythropoiesis in preclinical studies[15]. In a mouse model of anemia, treatment with IOX2 increased the expression of EPO and promoted the production of red blood cells. However, further studies are needed to determine the safety and efficacy of FIH inhibitors in humans.
Developing HIF modulators as therapeutics presents several challenges. One major concern is the potential for off-target effects, given the role of HIF in many cellular processes. For example, HIF inhibitors may also inhibit HIF-1α activity, which is involved in a range of physiological processes. Safety is also a concern, especially given the potential for HIF to promote tumor growth and metastasis in certain conditions.
Another challenge is the need for effective biomarkers to monitor treatment efficacy and safety. Currently available biomarkers have limitations in predicting clinical outcomes and monitoring treatment response. Therefore, developing more sensitive and specific biomarkers is a significant area of research in HIF modulation.
Despite these challenges, HIF modulators represent a promising avenue for treating a range of diseases. HIF-2α inhibitors have shown potential in treating renal cell carcinoma and are being evaluated in clinical trials. Other potential applications include ischemic diseases, such as stroke and myocardial infarction, and anemia associated with chronic kidney disease.
In addition to small molecule modulators, gene therapies targeting HIF are also gaining interest. Gene therapy approaches that deliver HIF-1α or HIF-2α genes may promote tissue repair and regeneration after ischemic injury.
The development of HIF modulators as therapeutics is a rapidly evolving field with significant potential. While challenges must be addressed to develop safe and effective HIF modulators, the potential benefits of these therapies in improving patient outcomes and reducing healthcare costs make it an active area of research and development.
Future directions for research on HIF modulators as therapeutics
In the field of HIF modulators as therapeutics, future research will prioritize several key areas to optimize drug efficacy and safety, develop combination therapies, and identify new disease indications. One major research focus will be the development of more selective HIF modulators that can target specific isoforms or components of the HIF pathway to minimize off-target effects and improve safety. Recent studies have identified HIF-2α as a potential therapeutic target in renal cell carcinoma, while HIF-1α is implicated in inflammatory diseases such as rheumatoid arthritis. Targeting these isoforms specifically may provide more effective and safer therapies for these diseases.
Understanding the mechanisms by which HIF modulators affect the HIF pathway and other signaling pathways is another critical area of research to optimize drug efficacy and minimize side effects. Recent studies suggest that HIF modulators may interact with other signaling pathways, such as mTOR and MAPK, to regulate cellular responses to hypoxia. This understanding may provide new opportunities for combination therapies that target multiple signaling pathways in disease.
Another important research direction is the development of combination therapies that target multiple components of the HIF pathway and other signaling pathways involved in disease pathogenesis. For example, the combination of PHD inhibitors with immunotherapy may provide a more effective approach to treating cancer by targeting both tumor growth and the immune response to tumors. Similarly, the combination of HIF-2α inhibitors with anti-angiogenic agents may be more effective in treating renal cell carcinoma.
Lastly, identifying new disease indications for HIF modulators will be a critical research focus. While HIF modulators have shown promise in treating cancer, anemia, and ischemic diseases, there may be other diseases and conditions where HIF modulation could be beneficial. Recent studies suggest that targeting HIF-1α may provide a new approach to treating neurodegenerative diseases such as Alzheimer’s and Parkinson’s.
Overall, continued research and development in HIF modulators as therapeutics is essential to realizing the potential of these drugs to improve patient outcomes for a wide range of diseases.