How to overcome the resistance of GBM to Temozolomide?
Abstract
As a new alkylating agent, Temozolomide is currently the only oral preparation for the treatment of glioma. It has the advantages of convenient administration, high patient compliance, good bioavailability, easy penetration of the blood-brain barrier, and increased radiotherapy sensitivity. Clinical studies have shown that TMZ not only has a good effect on glioma but also has a good application prospect for pituitary adenoma, central nervous system lymphoma, brain metastases, STS, and so on. However, large-scale clinical studies need to be further carried out to standardize and expand the clinical application of TMZ.
What is glioma?
Glioma is the most aggressive and common primary malignant tumor in the brain and other central nervous systems. It has the characteristics of high incidence, high recurrence rate, high degree of malignancy, high invasiveness, difficult treatment, poor clinical prognosis and high mortality, including astrocytoma, oligodendroglioma, ependymoma and some rare histological types. Among them, glioblastoma (GBM) has the highest degree of malignancy, accounting for more than half of all gliomas. At the same time, due to the rapid proliferation and high infiltration of glioblastoma, most GBM patients will relapse even after surgical resection and follow-up treatment. For patients newly diagnosed with GBM, postoperative radiotherapy combined with chemotherapy has been used as a conventional therapy. Temozolomide (TMZ) is an oral alkylating agent. Its anti-tumor activity was first discovered in 1987 and has been widely used as a first-line chemotherapy for GBM. Compared with radiotherapy alone, TMZ combined with radiotherapy can significantly prolong the median survival time of patients and improve the quality of life.
Introduction of temozolomide
TMZ is an alkylated imidazole tetrazine derivative with small molecular weight, stable structure, high lipophilicity, easy penetration of the blood-brain barrier, and high bioavailability. TMZ was approved by the FDA in 1995 for clinical use. It is the first alkylated drug approved for the clinical treatment of glioma. It is an oral chemotherapeutic drug that will not be hydrolyzed at normal gastric acid levels and can be stable. It is easily absorbed by the digestive tract after entering the human body and can quickly pass through the blood-brain barrier, so it is suitable for the treatment of glioma.
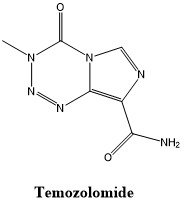
Fig 1. The structural formula of Temozolomide.
In recent years, many clinical trials have shown that TMZ plays an important role in the treatment of glioma and can significantly improve the survival of GBM patients. TMZ has relatively few side effects, mainly nausea, and vomiting, while hematological toxicity and hepatotoxicity occur less, which can significantly improve the quality of life of patients while prolonging the survival of glioma patients. Therefore, temozolomide is considered to be the first choice for the treatment of glioma.
How does temozolomide treat glioma?
After TMZ is absorbed into the systemic circulation through the digestive tract, it will hydrolyze and rapidly convert into active compounds at physiological pH to form MTIC, which will further hydrolyze into AIC and highly reactive electrophilic methyl diazonium cation. The unstable cation can react with DNA and histones, further releasing methyl groups, resulting in alkylation of DNA and histones. DNA alkylation causes base mismatches during DNA replication, resulting in DNA single-strand and double-strand breaks, causing DNA damage. Damaged DNA can cause cell cycle arrest and activate cell apoptosis pathways. Eventually, lead to the death of tumor cells.
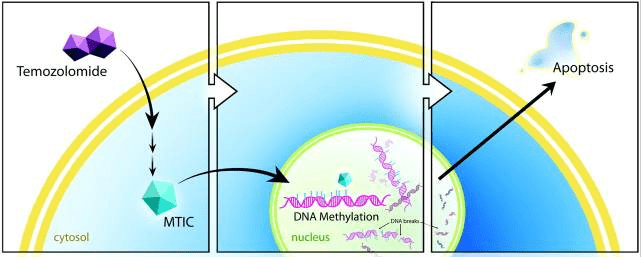
Fig 2. Schematic illustration of the proposed mechanism of temozolomide. Temozolomide is converted intracellularly into MTIC, which methylates DNA. Cellular repair mechanisms cannot adjust, resulting in DNA nicks and ultimately apoptosis [1].
Treatment status of temozolomide
TMZ has made gratifying achievements in the clinical research of glioma adjuvant therapy, but some glioma patients have congenital or acquired resistance to TMZ, which limits the clinical benefits of glioma patients. TMZ resistance often occurs in patients in the middle and late stages of chemotherapy, which seriously limits its therapeutic effect and leads to treatment failure. The resistance of glioma to TMZ involves a variety of mechanisms, including drug efflux transport mechanism, GSC, tumor cell autophagy, miRNA, abnormal cell signaling pathway, and DNA damage repair mechanism. Among them, DNA repair protein MGMT is considered to be one of the most important reasons for glioma resistance to TMZ. Therefore, reversing the drug resistance of glioma to TMZ is considered to be one of the keys to improving the quality of life and survival status of glioma patients.
The main strategies to overcome the resistance of GBM to TMZ
TMZ is the best chemotherapeutic drug for the treatment of GBM, but its drug resistance is a key problem to be solved. At present, many innovative treatment methods have emerged. According to the different drug resistance mechanisms of TMZ, different treatment strategies can be adopted to overcome the resistance of GBM to TMZ, and improve the quality of life of patients while improving the efficacy.
(1) Optimization of drug delivery methods. It can make TMZ more through the blood-brain barrier, such as targeted delivery of drugs through nanocarriers, and indoor and intracavitary administration methods have also been widely tested in clinical trials.
(2) Existing combination therapy. By combining high temperature with radiation, TMZ resistance can be overcome. Hyperthermia acts on TMZ-resistant glioma cells, which can make these cells more sensitive to TMZ and lead to cell death by down-regulating MGMT expression. Recent studies have shown that bevacizumab combined with TMZ is expected to be effective for progressive or recurrent GBM previously exposed to TMZ. Immune checkpoint inhibitors PD-1/PD-L1 inhibitors and CTLA-4 inhibitors have been used in a variety of tumors, including GBM, and several clinical studies are ongoing. Immunotherapy combined with chemotherapy may be one of the strategies to overcome TMZ drug resistance.
(3) The efficacy of TMZ was enhanced by combined therapy targeting drug resistance mechanisms. Inhibition of certain molecular targets associated with apoptosis and autophagy pathways, such as m TOR, PI3 K, and Akt, TMZ combined with these inhibitors as adjuvant chemotherapy drugs can improve the therapeutic effect. PARP is one of the most concerned BER enzymes. Some preclinical studies have shown that the combination of TMZ and PARP inhibitors is effective for IDH mutant gliomas and can enhance the efficacy of TMZ. In addition, studies have found that JNK inhibitors can enhance the sensitivity of tumors to TMZ by regulating the expression of MGMT, and MEK inhibitors can enhance TMZ chemotherapy targeting GSCs. The combination of TMZ and these drugs may be a potential strategy to overcome drug resistance.
(4) RNA interference technology. It is a gene regulation strategy that can reduce the expression of many proteins related to GBM resistance to TMZ. This strategy can be used for different genes involved in the mechanism of GBM resistance to TMZ. These targets are identified by genetic screening to find out the out-of-control genes of each individual, such as miRNAs targeting the GBM signaling pathway, down-regulation of carcinogenic miRNAs through miRNA sponges or antisense oligonucleotides, and repair of tumor suppressor miRNAs with synthetic mimics or gene replacement therapy. This personalized medical treatment can provide a treatment plan for each patient. It is one of the most promising strategies to overcome TMZ resistance in glioma.