How does Cenicriviroc Treat Nonalcoholic Steatohepatitis ?
Abstract
Non-alcoholic steatohepatitis (NASH) is a rapidly growing disease and a leading cause of liver failure worldwide. Currently, there are no approved therapies for NASH, and the standard of care is weight loss, exercise, and dietary modification. Liver fibrosis, which can lead to cirrhosis and hepatocellular carcinoma, is also a hallmark of NASH. Cenicriviroc (CVC) is an oral drug that targets the chemokine receptors CCR2 and CCR5 involved in the recruitment and activation of inflammatory cells in the liver. This review provides a comprehensive overview of preclinical and clinical studies on CVC, its mechanism of action, and its potential therapeutic use for NASH and liver fibrosis.
Introduction to Non-alcoholic Steatohepatitis (NASH) and Liver Fibrosis
The incidence of non-alcoholic fatty liver disease (NAFLD), a chronic liver condition, is on the rise globally, with a prevalence of around 25%. NAFLD covers a range of liver diseases, from simple steatosis to non-alcoholic steatohepatitis (NASH), which can lead to liver fibrosis, cirrhosis, and hepatocellular carcinoma. Despite its growing prevalence, there are currently no approved pharmacological treatments for NASH, and the available standard of care for liver fibrosis patients remains limited.
Cenicriviroc (CVC) is a novel dual C-C chemokine receptor type 2 and 5 (CCR2/CCR5) antagonist that has shown promising results in clinical trials for the treatment of NASH and liver fibrosis. CVC works by reducing inflammation and fibrosis in the liver, which are two key components of NASH progression.
Cenicriviroc: A Potential Therapeutic Agent for Non-Alcoholic Steatohepatitis (NASH)
Cenicriviroc ( CVC ) is a dual antagonist of CCR2 and CCR5, two chemokine receptors involved in the recruitment of inflammatory cells to the liver. As a potential therapeutic drug for NASH, CVC has shown good results in clinical trials. Compared with placebo, liver inflammation and fibrosis are significantly reduced.
The results showed that compared with patients who continued to receive placebo, patients who did not experience stage 1 or better fibrosis improvement within 1 year of placebo treatment improved fibrosis after switching to cenicriviroc.
The researchers observed a higher proportion of cenicriviroc patients with fibrosis reaching stage 2 or better improvement. Patients who took cenicriviroc in the second year continued to experience more pronounced decreases in inflammatory biomarkers, including interleukin-1β, interleukin-8, high-sensitivity C-reactive protein, and fibrinogen.
Ratzu reported that cenicriviroc was safe and well tolerated within two years without new or unexpected side effects. There were no drug-related serious adverse events and no patient died. The results of exploratory analysis ‘ confirmed the anti-fibrotic activity of cenicriviroc in adult NASH and liver fibrosis patients ‘, the researchers concluded.
Compared with other drugs, CVC has a unique mechanism of action and may provide a safer alternative with fewer side effects.
Figure 1 Structure of Cenicriviroc
Cenicriviroc: Mechanism of Action and Clinical Development
Cenicriviroc (CVC) is an oral drug designed to target chemokine receptors CCR2 and CCR5. These receptors are critical in the recruitment and activation of inflammatory cells within the liver. Monocytes and macrophages express CCR2, while T-cells and macrophages express CCR5. In studies involving animal models of non-alcoholic steatohepatitis (NASH), CVC has been demonstrated to decrease liver inflammation, fibrosis, and steatosis by inhibiting chemokine signaling and reducing the infiltration of inflammatory cells within the liver.
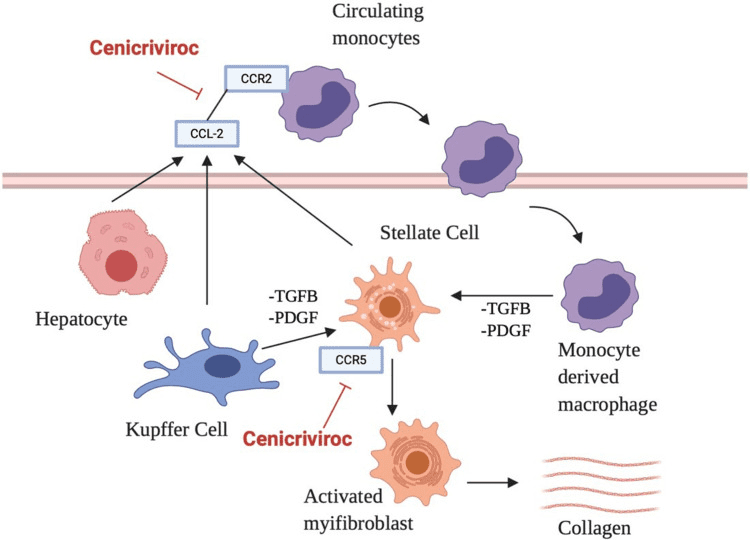
Figure 2 [5] Cenicriviroc mechanism of action.
Although preclinical studies have shown promise, using CVC for treating NASH and liver fibrosis has limitations that need addressing. Establishing CVC’s safety and efficacy requires longer-term studies. Further research is necessary to optimize dosing, treatment duration, and explore the potential of combining CVC with other drugs to improve its effectiveness.
Safety and Tolerability of Cenicriviroc in the Treatment of NASH
Several clinical trials have examined the safety and tolerability of Cenicriviroc (CVC) in treating non-alcoholic steatohepatitis (NASH), a liver disease characterized by inflammation and damage due to fat buildup in the liver. These trials varied in dosages and duration of treatment.
CVC was generally found to be well-tolerated and safe. Common side effects included mild to moderate gastrointestinal symptoms like nausea and diarrhea, which usually resolved without intervention. No severe adverse events related to CVC treatment were reported.
Modest increases in serum creatinine levels, a marker of kidney function, were observed in some patients taking CVC. However, these changes were reversible upon stopping treatment and didn’t cause any negative clinical outcomes.
In conclusion, CVC appears to be a promising medication for NASH treatment with a favorable safety and tolerability profile.
Efficacy of Cenicriviroc in the Treatment of NASH and Liver Fibrosis
Compared to other therapeutic agents, Cenicriviroc (CVC) has demonstrated potential as a treatment for non-alcoholic steatohepatitis (NASH) and liver fibrosis due to its distinct mechanism of action. CVC is a dual antagonist of chemokine receptors CCR2 and CCR5, which are involved in the recruitment of inflammatory cells to the liver. By inhibiting these receptors, CVC can effectively reduce liver inflammation and fibrosis. This mechanism sets it apart from other agents that target different pathways, such as insulin resistance or oxidative stress.
Furthermore, Cenicriviroc (CVC) has displayed promising outcomes in clinical trials, revealing considerable decreases in liver inflammation and fibrosis in NASH patients after a year of treatment in comparison to placebo. Nevertheless, additional research is necessary to establish the ideal dosage and treatment duration, as well as the long-term safety and effectiveness of CVC. Despite these limitations, CVC’s distinctive mechanism of action and encouraging clinical outcomes suggest its potential as a treatment option for NASH and liver fibrosis.
Compared to other agents commonly used to treat NASH, such as vitamin E, pioglitazone, and obeticholic acid (OCA), Cenicriviroc (CVC) offers a unique mechanism of action. While vitamin E has been shown to improve liver histology, its long-term safety remains uncertain. Pioglitazone can reduce inflammation and fibrosis, but it is associated with weight gain and bone fractures. OCA, though approved for NASH treatment, can cause itching and increase the risk of cardiovascular events. In contrast, CVC inhibits the recruitment of inflammatory cells to the liver, reducing liver inflammation and fibrosis. Further research is needed to determine the optimal dosing and long-term safety and efficacy of CVC.
Future Directions for the Development of Cenicriviroc in the Treatment of NASH and Related Disorders
Cenicriviroc is a drug that has been studied for the treatment of nonalcoholic steatohepatitis ( NASH ) and liver fibrosis. The development of Cenicriviroc for the treatment of NASH and related diseases is a promising research field.
Next, we need to determine the optimal dose and treatment time of CVC to determine a more effective and safe method ; at the same time, it is also necessary to better understand the mechanism of CVC in NASH and related diseases, and to establish the long-term safety and effectiveness of CVC through further clinical trials.
In general, CVC has several promising development directions in the treatment of NASH and related diseases, and further research is needed to optimize the use of this preparation.
References
- Tacke F. Cenicriviroc for the treatment of non-alcoholic steatohepatitis and liver fibrosis. Expert Opin Investig Drugs. 2018 Mar;27(3):301-311. doi: 10.1080/13543784.2018.1442436. Epub 2018 Feb 22. PMID: 29448843.
- Wong VW. Current Prevention and Treatment Options for NAFLD. Adv Exp Med Biol. 2018;1061:149-157. doi: 10.1007/978-981-10-8684-7_12. PMID: 29956213.
- Li M, Chen L, Gao Y, Li M, Wang X, Qiang L, Wang X. Recent advances targeting C-C chemokine receptor type 2 for liver diseases in monocyte/macrophage. Liver Int. 2020 Dec;40(12):2928-2936. doi: 10.1111/liv.14687. Epub 2020 Oct 21. PMID: 33025657.
- Gawrieh S, Chalasani N. Emerging Treatments for Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Clin Liver Dis. 2018 Feb;22(1):189-199. doi: 10.1016/j.cld.2017.08.013. Epub 2017 Oct 10. PMID: 29128056.
- Diaz Soto MP, Lim JK. Evaluating the Therapeutic Potential of Cenicriviroc in the Treatment of Nonalcoholic Steatohepatitis with Fibrosis: A Brief Report on Emerging Data. Hepat Med. 2020 Aug 13;12:115-123. doi: 10.2147/HMER.S230613. PMID: 32884369; PMCID: PMC7434517.
- Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018 Mar;53(3):362-376. doi: 10.1007/s00535-017-1415-1. Epub 2017 Dec 16. PMID: 29247356; PMCID: PMC5847174.