How Chemokines Shape the Future of Multiple Myeloma Therapy
Abstract
Multiple myeloma (MM) remains an incurable plasma cell malignancy, driven not only by genetic alterations but also by complex interactions within the tumor microenvironment. Recent research highlights the critical role of chemokines—small signaling proteins—in promoting MM progression, metastasis, bone destruction, and immune evasion. Some chemokines act as pro-tumor agents, aiding drug resistance and dissemination, while others support anti-tumor immunity by recruiting cytotoxic immune cells. This blog explores emerging insights into chemokine signaling in MM, the dual nature of specific chemokine axes, and how targeted modulation of these pathways could enhance the efficacy of therapies like CAR-T cells. Ongoing clinical trials and novel therapeutic strategies point to chemokine modulation as a promising frontier in personalized MM treatment.
Introduction: A New Frontier in Multiple Myeloma Therapy
Multiple myeloma (MM) is a complex and aggressive blood cancer that originates from malignant plasma cells in the bone marrow. Despite significant advances in treatment—including proteasome inhibitors, immunomodulatory drugs, monoclonal antibodies, and CAR-T cell therapy—MM remains largely incurable. Most patients eventually relapse, and treatment resistance often emerges, posing significant challenges for long-term disease control and quality of life.
As researchers dig deeper into the biology of MM, it is becoming increasingly clear that the tumor microenvironment (TME) plays a central role in disease progression. One of the most crucial, yet underrecognized, components of the TME is the chemokine network. Chemokines are small signaling proteins that regulate immune cell movement, tissue repair, and inflammation. In the context of MM, however, these molecules can be hijacked by cancer cells to facilitate tumor growth, immune evasion, bone destruction, and even metastasis to sites outside the bone marrow (a phenomenon known as extramedullary disease).
A growing body of research highlights the dual nature of chemokines: while some support immune surveillance and anti-tumor responses, others are co-opted by malignant cells to suppress immunity and foster a pro-tumor niche. This duality opens up exciting opportunities in precision oncology. By understanding which chemokine-receptor pairs are aiding disease and which are fighting it, researchers are uncovering new therapeutic targets that could redefine MM treatment paradigms.
In particular, targeting chemokines such as CCL2, CCL3, and CXCL12—or their receptors like CCR1, CCR2, and CXCR4—has shown promise in preclinical and clinical settings. Conversely, enhancing the action of anti-tumor chemokines such as CCL19 or CXCL10 may improve the performance of existing therapies like CAR-T cells.
This blog series will explore these new findings, highlighting the pivotal role of chemokines in MM biology and their therapeutic potential. The future of MM treatment may lie not only in targeting the tumor cells themselves but also in reshaping the signals they depend on to thrive.
Chemokines at the Heart of MM Progression
Multiple myeloma (MM) is not solely driven by genetic mutations; it is also heavily influenced by the tumor microenvironment (TME)—a dynamic network of immune cells, stromal cells, and signaling molecules. At the center of this interplay lies a group of small, secreted proteins known as chemokines, which play a decisive role in how MM cells grow, survive, and spread.
Chemokines are essential for immune cell migration and communication. However, MM cells can exploit this signaling system to their advantage. For example, CCL2, CCL3, and CCL5 are overexpressed in the myeloma microenvironment and are strongly linked to drug resistance, immune suppression, and poor prognosis. These chemokines attract tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs), creating an immunosuppressive environment that shields MM cells from chemotherapy-induced apoptosis.
The CCL3-CCR1 axis plays a particularly destructive role. CCL3 disrupts red blood cell formation by impairing GATA1 expression in hematopoietic progenitors, contributing to MM-associated anemia. Simultaneously, this pathway enhances the migration of MM cells from the bone marrow into the bloodstream, facilitating extramedullary disease and metastasis.
Bone lesions—another hallmark of MM—are also linked to chemokines. CXCL7 and CXCL8, for instance, drive the recruitment and activation of osteoclasts, leading to accelerated bone resorption. In particular, CXCL7 bioavailability is increased by MMP-13, a matrix metalloproteinase released by stromal cells. This enhances osteoclastogenesis and worsens patient outcomes.
Additionally, CXCL12 (also known as SDF-1) is crucial for MM cell adhesion to the bone marrow niche. By binding to CXCR4, it enhances MM cell survival and drug resistance. Elevated CXCL12 expression is also associated with extramedullary dissemination of plasma cells.
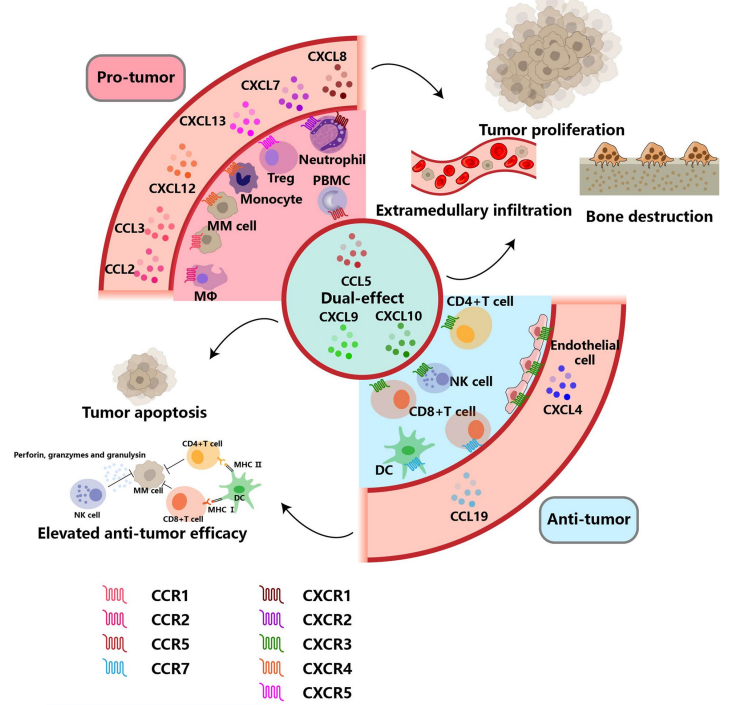
Fig. 1 The chemokine network in multiple myeloma
In essence, pro-tumor chemokines form a signaling web that helps MM cells escape immune destruction, resist treatment, and cause systemic damage. Understanding this network opens the door to therapeutic strategies that not only target the cancer cells but also dismantle the supportive signals they depend on.
Turning the Tide: Anti-Tumor Chemokines and Immune Reinforcement
While many chemokines fuel the progression of multiple myeloma (MM), a subset of these signaling molecules plays a protective role in anti-tumor immunity. These anti-tumor chemokines help orchestrate the immune response by attracting and activating cytotoxic immune cells such as natural killer (NK) cells, cytotoxic T lymphocytes (CD8+ T cells), and dendritic cells (DCs). Understanding and harnessing these chemokines presents a promising strategy to counteract the disease and enhance the effectiveness of immunotherapies like CAR-T cells.
Among the most studied anti-tumor chemokines in MM is CXCL10, also known as interferon gamma-induced protein 10 (IP-10). CXCL10 binds to CXCR3 receptors on immune cells, promoting chemotaxis, cytotoxicity, and proliferation. Recent studies have shown that CXCL10 not only improves the recruitment of immune effector cells to the tumor site but also reduces PD-1 expression on CAR-T cells, thereby limiting exhaustion and prolonging their function. In MM patients receiving CAR-T therapy, higher baseline levels of CXCL10 correlate with better clinical outcomes.
CCL19 is another key chemokine that facilitates the migration and retention of DCs and T cells within the tumor microenvironment. In preclinical models, arming CAR-T cells with CCL19 and IL-7 (also known as “7×19 CAR-T cells”) has led to enhanced immune infiltration, improved CAR-T persistence, and more robust tumor clearance. This dual cytokine-chemokine strategy not only boosts cytotoxicity but also supports the generation of stem cell-like memory T cells (Tscms), which are critical for sustained response.
Additionally, CXCL4, also called platelet factor 4 (PF-4), has been shown to inhibit MM cell survival by promoting STAT3 repression via SOCS3 upregulation. High serum levels of CXCL4 are associated with favorable prognosis in newly diagnosed patients, making it a potential biomarker as well as a therapeutic adjunct.
Together, these chemokines offer new avenues for immunomodulation in MM. Whether used as biomarkers, immune boosters, or genetic payloads in engineered cell therapies, anti-tumor chemokines are proving to be powerful allies in turning the tide against myeloma.
Targeting Chemokine Pathways: From Bench to Bedside
With chemokines playing such a central role in the development, progression, and treatment resistance of multiple myeloma (MM), researchers have begun to turn these signaling molecules into therapeutic targets. This approach—disrupting pro-tumor chemokine axes or enhancing anti-tumor ones—has already transitioned from laboratory discovery to early clinical application, with promising results.
One of the most extensively studied chemokine pathways in MM is CXCL12-CXCR4. This axis promotes MM cell survival, adhesion to the bone marrow niche, and chemoresistance. Plerixafor (AMD3100), a CXCR4 antagonist, is currently the only chemokine-targeting drug approved for use in MM, primarily to mobilize hematopoietic stem cells for autologous transplantation. However, clinical trials have also shown its ability to sensitize MM cells to bortezomib, a frontline proteasome inhibitor, by disrupting the protective microenvironment.
New agents such as motixafortide (BKT140) and ulocuplumab have entered clinical trials, demonstrating efficacy in mobilizing stem cells and improving response rates when combined with standard MM therapies. Another approach, using olaptesed pegol, targets the ligand CXCL12 itself rather than its receptor. This compound has shown promising results in relapsed/refractory MM patients when combined with dexamethasone.
Beyond CXCL12, researchers are investigating other chemokine targets. The CCL3-CCR1 axis, implicated in MM dissemination and anemia, has become a focus for CCR1 antagonists such as BX471 and CCX9588. These agents aim to block MM cell migration and reduce the formation of immunosuppressive macrophages. While many of these inhibitors remain in preclinical or early-phase trials, they offer novel avenues for intervention.
Moreover, BTK inhibitors like ibrutinib have been shown to suppress CXCL13 expression and its downstream osteolytic and chemoresistant effects. EGFR inhibitors such as gefitinib are also being tested for their ability to reduce CXCL8-induced bone damage.
These targeted strategies represent a shift in MM therapy—moving from solely attacking malignant cells to disrupting the communication channels they rely on for survival. As these therapies advance through the clinical pipeline, they hold the potential to transform patient outcomes.
What’s Next: The Future of Chemokine-Based MM Treatment
As our understanding of multiple myeloma (MM) deepens, chemokines have emerged as more than just bystanders in disease progression—they are active participants and potentially powerful therapeutic targets. Yet, as the authors of the reviewed study suggest, most chemokine-targeting strategies remain in early development. The challenge and opportunity now lie in translating these insights into clinical breakthroughs.
Currently, CXCL12-CXCR4 remains the most clinically advanced chemokine axis in MM treatment. While plerixafor and motixafortide have established efficacy in stem cell mobilization and show potential as chemosensitizers, their success has inspired interest in developing new inhibitors, antibodies, and modulators for less-explored chemokines like CCL3, CXCL13, and CXCL8. These targets lie beneath the surface of what researchers metaphorically describe as the “iceberg” of chemokine modulation—promising but still submerged in preclinical research.
One of the most exciting frontiers is the integration of chemokine signaling into immunotherapies. By engineering CAR-T cells to secrete anti-tumor chemokines like CCL19 or CXCL10, scientists can potentially improve cell migration, persistence, and tumor-killing activity. Early studies show that this approach can reduce T cell exhaustion and improve outcomes in patients with refractory disease. As more trials assess chemokine-enhanced CAR-T constructs, this strategy may redefine immune cell-based therapies in MM.
Another promising direction is the use of chemokines as biomarkers. For example, high levels of CXCL10 and PF-4 (CXCL4) have been linked to better prognosis, while elevated CCL3 and CXCL8 are associated with anemia and bone destruction. Monitoring these chemokines could inform patient stratification, treatment planning, and real-time evaluation of therapy response.
However, the path ahead is not without challenges. Chemokines often interact with multiple receptors, and blocking one receptor may result in compensatory signaling through another. This complexity demands combination approaches, personalized targeting, and continued investigation into the downstream pathways influenced by chemokine activity.
The future of MM therapy may hinge on our ability to move beyond targeting cancer cells alone and instead reshape the ecosystem they rely on. As new modulators surface and clinical strategies mature, chemokine-based therapies could become a cornerstone of personalized, durable MM treatment.
References
Du, J., Lin, Z., Fu, X. H., Gu, X. R., Lu, G., & Hou, J. (2024). Research progress of the chemokine/chemokine receptor axes in the oncobiology of multiple myeloma (MM). Cell Communication and Signaling, 22(177).
https://doi.org/10.1186/s12964-024-01544-7
van de Donk, N. W. C. J., Pawlyn, C., & Yong, K. L. (2021). Multiple myeloma. The Lancet, 397(10272), 410–427.
https://doi.org/10.1016/S0140-6736(21)00135-5
Grifith, J. W., Sokol, C. L., & Luster, A. D. (2014). Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annual Review of Immunology, 32, 659–702.
https://doi.org/10.1146/annurev-immunol-032713-120145
Xu, R., et al. (2019). CCL2 promotes macrophages-associated chemoresistance via MCPIP1 dual catalytic activities in multiple myeloma. Cell Death & Disease, 10(781).
https://doi.org/10.1038/s41419-019-2004-1
Liu, L., et al. (2020). Multiple myeloma hinders erythropoiesis and causes anemia owing to high levels of CCL3 in the bone marrow microenvironment. Scientific Reports, 10(20508).
https://doi.org/10.1038/s41598-020-77533-3
Lo, C. H., et al. (2021). Host-derived matrix metalloproteinase-13 activity promotes multiple myeloma-induced osteolysis and reduces overall survival. Cancer Research, 81(9), 2415–2428.
https://doi.org/10.1158/0008-5472.CAN-20-3580
Ren, Z., et al. (2021). The CXCL12gamma chemokine immobilized by heparan sulfate on stromal niche cells controls adhesion and mediates drug resistance in multiple myeloma. Journal of Hematology & Oncology, 14(11).
https://doi.org/10.1186/s13045-021-01042-5
Liu, T., et al. (2022). IP-10 enhances the amplification capacity and antitumor activity of CAR-T cells in vitro and could influence positive outcomes in MM patients treated with CAR-T cell therapy. International Immunopharmacology, 112, 109253.
https://doi.org/10.1016/j.intimp.2022.109253
Duan, D., et al. (2021). The BCMA-targeted fourth-generation CAR-T cells secreting IL-7 and CCL19 for therapy of refractory/recurrent multiple myeloma. Frontiers in Immunology, 12, 609421.
https://doi.org/10.3389/fimmu.2021.609421
Pei, L., et al. (2013). Platelet factor 4 induces cell apoptosis by inhibition of STAT3 via up-regulation of SOCS3 expression in multiple myeloma. Haematologica, 98(2), 288–295.
https://doi.org/10.3324/haematol.2012.067330
Ghobrial, I. M., et al. (2019). Phase I/II trial of the CXCR4 inhibitor plerixafor in combination with bortezomib as a chemosensitization strategy in relapsed/refractory multiple myeloma. American Journal of Hematology, 94(11), 1244–1253.
https://doi.org/10.1002/ajh.25606
Ludwig, H., et al. (2017). Olaptesed pegol, an anti-CXCL12/SDF-1 Spiegelmer, alone and with bortezomib-dexamethasone in relapsed/refractory multiple myeloma: a phase IIa study. Leukemia, 31(4), 997–1000.
https://doi.org/10.1038/leu.2016.296
Adachi, K., et al. (2018). IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nature Biotechnology, 36(4), 346–351.
https://doi.org/10.1038/nbt.4086
Zeissig, M. N., et al. (2020). Expression of the chemokine receptor CCR1 promotes the dissemination of multiple myeloma plasma cells in vivo. Haematologica, 106(12), 3176–3187.




