Histamine in the Brain: A Hidden Key to Unlocking New Depression Treatments
Abstract
Despite major advances in antidepressant pharmacotherapy, a significant proportion of patients with major depressive disorder (MDD) fail to respond to conventional treatments targeting serotonin, dopamine, and norepinephrine. Emerging research highlights histamine—a monoamine traditionally associated with allergy and immune function—as a powerful neuromodulator within the central nervous system. This blog explores the role of the histaminergic system and its four receptors (H1R–H4R) in the regulation of mood, cognition, circadian rhythm, and neuroinflammation. Special attention is given to H3 and H1 receptor antagonists, which have demonstrated antidepressant-like effects in preclinical studies by modulating neurotransmitter networks and immune responses. The potential of histamine-based therapies to complement or replace existing antidepressant strategies signals a promising frontier in psychiatric medicine.
Rethinking Depression Beyond Serotonin: The Overlooked Role of Histamine
Major depressive disorder (MDD) is a complex and debilitating psychiatric condition that affects over 250 million people globally. It is projected to become one of the leading causes of disability worldwide, with significant consequences for both individuals and society. Despite decades of research and the widespread use of antidepressant medications, a substantial number of patients—up to 50%—fail to achieve full remission with current treatments (Thase et al., 2010). This persistent treatment gap highlights the urgent need for new therapeutic targets and a deeper understanding of depression’s neurobiology.
For years, the dominant neurochemical theory of depression has been the monoamine hypothesis, which focuses on deficiencies in serotonin (5-HT), norepinephrine (NE), and dopamine (DA). These neurotransmitters form the foundation of most modern antidepressant drugs, including SSRIs and SNRIs. However, an often-overlooked player in this family of monoamines is histamine—a neurotransmitter more commonly associated with immune responses and allergies than with mental health.
Emerging evidence suggests that histamine plays a crucial role in brain function, influencing mood, cognition, circadian rhythm, and even neuroinflammation. Histamine operates through four receptors—H1R, H2R, H3R, and H4R—all of which have diverse distributions and functions in the central nervous system. While H1 and H2 receptors are better known for their roles in sleep and gastric acid secretion, H3 and H4 receptors are gaining attention for their involvement in psychiatric disorders, including depression.
Importantly, histamine is not working in isolation. It interacts closely with the same neurotransmitter systems already implicated in depression. For instance, H3 receptors modulate the release of serotonin and dopamine, while also forming receptor complexes with NMDA receptors—critical in learning, memory, and neuroplasticity.
This growing body of research is challenging the serotonin-centric view of depression and prompting scientists to explore the histaminergic system as a novel target for antidepressant therapy. Recognizing histamine’s influence on emotional and cognitive processes could open the door to more effective, multi-modal treatments for depression—particularly for patients who do not respond to conventional medications.
The Brain’s Histamine System: More Than an Allergy Response
When most people hear the word “histamine,” they immediately think of sneezing, itchy eyes, and allergic reactions. But beyond its well-known role in the immune system, histamine is also a powerful neurotransmitter in the brain—one that plays critical roles in regulating mood, cognition, sleep, appetite, and neuroinflammation.
In the central nervous system (CNS), histamine is synthesized by neurons located in the tuberomammillary nucleus (TMN) of the posterior hypothalamus. These neurons send widespread projections to nearly every major brain region, including the cortex, hippocampus, amygdala, and basal forebrain—areas heavily implicated in emotional processing and depression. Histamine exerts its effects via four G-protein-coupled receptors: H1R, H2R, H3R, and H4R, each with distinct localization and functions in the CNS.
H1 receptors (H1R) are broadly distributed in the cortex, thalamus, and hippocampus. They modulate wakefulness, attention, locomotion, and thermoregulation. H1R antagonists are widely used as sedatives due to their ability to cross the blood-brain barrier and induce drowsiness.
H2 receptors (H2R), although more famous for their role in gastric acid secretion, are also found in the hippocampus and cortex, where they regulate circadian rhythm and learning. Their psychiatric roles, however, are still not well established.
H3 receptors (H3R) are particularly significant in the context of depression. These receptors function as autoreceptors and heteroreceptors, inhibiting the release of histamine and other neurotransmitters like serotonin, dopamine, and glutamate. This makes H3R a promising target for mood disorder treatment.
H4 receptors (H4R) were the last to be discovered and are mainly expressed on immune cells. Although their presence in neurons is controversial, emerging evidence suggests they may influence neuroinflammatory responses relevant to depression.
What distinguishes histamine from other neurotransmitters is its dual role in both the nervous and immune systems, making it a potential bridge between inflammation and mood regulation. Understanding the functional diversity of histamine receptors in the brain is crucial as researchers consider them not just for allergy relief, but as viable targets for antidepressant development.
Histamine’s Crosstalk with Key Neurotransmitters in Depression
One of the most compelling reasons to consider histamine as a central player in depression is its extensive interaction with other neurotransmitter systems already linked to mood disorders. Histamine, via its H3 receptors (H3Rs), functions as a presynaptic modulator, regulating the release of multiple neurotransmitters including serotonin (5-HT), dopamine (DA), glutamate (GLU), and melanin-concentrating hormone (MCH)—all of which are implicated in the pathophysiology of major depressive disorder (MDD).
H3Rs are auto- and heteroreceptors predominantly found in the cortex, hippocampus, and striatum. Their activation inhibits histamine synthesis and release while also dampening the signaling of 5-HT and DA. For instance, H3R antagonists have been shown to enhance serotonin neuron excitability, which may help explain their antidepressant-like effects in animal models (Spaethling et al., 2014). This positions H3R antagonists as functional analogs to SSRIs, but potentially with broader neurotransmitter effects.
The interaction between dopamine and histamine is equally significant. Studies have identified D1R-H3R heteromers, specialized receptor complexes that link dopaminergic and histaminergic signaling through the MAPK pathway. These complexes are enriched in the striatum, a region involved in motivation and reward—key processes disrupted in depression (Moreno et al., 2011). Interestingly, mice lacking H3Rs show diminished dopaminergic responses, highlighting the receptor’s role in dopamine-dependent behavior.
Histamine also interacts with NMDA-type glutamate receptors, forming a triad with D1R and H3R in certain brain regions. Antagonizing H3Rs appears to reduce excitotoxicity, offering potential neuroprotective effects in addition to mood stabilization (Rodríguez-Ruiz et al., 2017).
Finally, melanin-concentrating hormone (MCH)—which promotes sleep and suppresses arousal—is directly inhibited by histamine via H3R. Since increased MCH activity is associated with depressive-like behavior, this antagonism further supports the role of histamine in mood regulation.
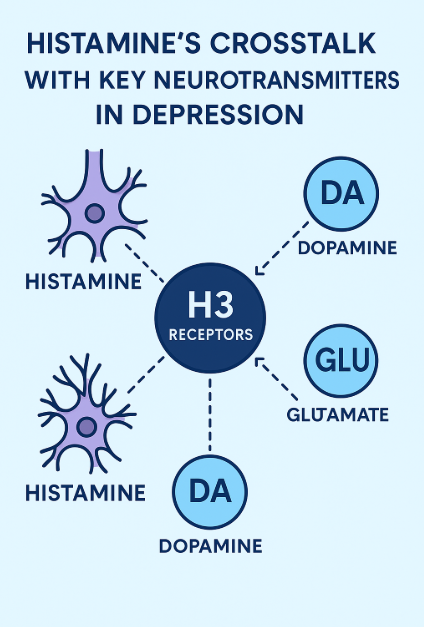
FIGURE 1. Histamine H3 Receptors and Mood
Through this multi-transmitter network, histamine emerges not just as a side player, but as a central integrator of emotional and cognitive circuits. By modulating serotonin, dopamine, glutamate, and MCH, the histaminergic system holds promise for developing multi-targeted antidepressant therapies.
Histamine and the Immune System: The Inflammation Link in Depression
An expanding body of research has revealed a profound connection between immune dysregulation and major depressive disorder (MDD). Among the key players at this intersection of neurology and immunology is histamine, a monoamine neurotransmitter traditionally known for mediating allergic responses. In recent years, histamine has emerged as a critical modulator of neuroinflammation, an increasingly recognized contributor to depression.
Histamine operates through four receptors—H1R, H2R, H3R, and H4R—each influencing immune function differently. Notably, H1R and H4R are found on microglia, the brain’s resident immune cells. When activated, these receptors can trigger the release of proinflammatory cytokines such as IL-6 and TNF-α, which are commonly elevated in individuals with depression. Studies have demonstrated that histamine can stimulate microglial activation via H1R and H4R through MAPK and PI3K/Akt–NF-κB signaling pathways, leading to a neuroinflammatory state linked to depressive behavior.
Interestingly, histamine also appears to play a regulatory role, capable of both exacerbating and suppressing inflammation depending on the context. For example, H4R activation can lead to either pro- or anti-inflammatory outcomes depending on the disease model. This duality positions the histaminergic system as a dynamic immunomodulator, influencing both the onset and resolution of inflammatory responses in the brain.
Beyond microglia, mast cells, another source of histamine, also reside in the brain and contribute to emotional regulation. Animal studies have shown that mast cell-deficient mice exhibit heightened anxiety and depressive-like behavior, which can be reversed by antidepressants. This suggests that mast cell-derived histamine may support resilience to stress and emotional dysregulation.
Moreover, the blood-brain barrier (BBB)—a critical interface between the peripheral immune system and the CNS—is sensitive to histamine. Elevated histamine levels can increase BBB permeability, potentially allowing peripheral inflammatory molecules to enter the brain and amplify depressive symptoms.
Altogether, the histaminergic system acts as a bridge between immune activity and brain function. By modulating cytokine production, microglial behavior, and BBB integrity, histamine is uniquely positioned to influence the inflammatory component of depression, making its receptors attractive candidates for novel therapeutic strategies.
Therapeutic Horizons: Histamine Receptor Antagonists as Antidepressants
As our understanding of depression evolves beyond the traditional monoamine hypothesis, the histaminergic system is drawing increasing interest as a novel therapeutic target. While histamine has long been associated with allergic reactions and gastric regulation, recent studies suggest that histamine receptor antagonists—particularly H1R and H3R antagonists—may offer antidepressant benefits.
Among the most promising findings are those involving H3R antagonists, which modulate the release of key neurotransmitters such as serotonin, dopamine, and glutamate—each central to mood regulation. Preclinical studies have shown that compounds like ciproxifan and clobenpropit, both H3R antagonists, reduce depression-like behaviors in animal models. These effects are believed to occur through normalization of stress-related markers like BDNF, corticosterone, and CRH, along with enhancement of hippocampal neuroplasticity.
Another compound, pitolisant, an H3R inverse agonist currently approved for treating narcolepsy, has shown potential to counteract antipsychotic-induced depressive symptoms in rodents. Importantly, these effects are achieved without the sedation typically associated with histamine-targeting drugs, making H3R antagonists an appealing class for further investigation.
Beyond H3R, H1R antagonists such as clemastine have also demonstrated antidepressant-like effects in mice. Clemastine appears to alleviate depressive symptoms by suppressing microglial inflammation in the hippocampus—a region crucial for mood and memory. Interestingly, some SSRIs like paroxetine have been found to upregulate histamine-related enzymes and receptors, indicating that the histaminergic system may even mediate part of the therapeutic response to existing antidepressants.
However, caution is warranted. Not all antihistamines are beneficial. For example, case reports have suggested that cetirizine, a second-generation H1R antagonist, may induce depressive symptoms in rare cases. This underscores the importance of receptor specificity, blood-brain barrier permeability, and pharmacodynamic context in developing histamine-based therapeutics.
Despite strong preclinical evidence, clinical trials specifically targeting histamine receptors for depression are still lacking. This presents an exciting opportunity for drug development. Given histamine’s central role in neurotransmission, immune regulation, and sleep—three pillars of mood stability—future antidepressant strategies could greatly benefit from targeting this underappreciated system.
References
Panula, P., Chazot, P. L., Cowart, M., Gutzmer, R., Leurs, R., Liu, W. L., … & Haas, H. L. (2015). International Union of Basic and Clinical Pharmacology. XCVIII. Histamine receptors. Pharmacological Reviews, 67(3), 601–655.
https://doi.org/10.1124/pr.114.010249
Qian, H., Shu, C., Xiao, L., & Wang, G. (2022). Histamine and histamine receptors: Roles in major depressive disorder. Frontiers in Psychiatry, 13, 825591.
https://doi.org/10.3389/fpsyt.2022.825591
Thase, M. E., Nierenberg, A. A., Vrijland, P., van Oers, H. J., Schutte, A. J., & Simmons, J. H. (2010). Remission with mirtazapine and selective serotonin reuptake inhibitors: A meta-analysis of individual patient data. International Clinical Psychopharmacology, 25(4), 189–198.
https://doi.org/10.1097/YIC.0b013e328330adb2
Benarroch, E. E. (2010). Histamine in the CNS: multiple functions and potential neurologic implications. Neurology, 75(16), 1472–1479.
https://doi.org/10.1212/WNL.0b013e3181f884b1
Panula, P., Nuutinen, S. (2013). The histaminergic network in the brain: basic organization and role in disease. Nature Reviews Neuroscience, 14(7), 472–487.
https://doi.org/10.1038/nrn3526
Schneider, E. H., Neumann, D., & Seifert, R. (2014). Modulation of behavior by the histaminergic system: Lessons from H1R- and H2R-deficient mice. Neuroscience & Biobehavioral Reviews, 42, 252–266.
https://doi.org/10.1016/j.neubiorev.2014.03.009
Seifert, R., & Strasser, A. (2013). Molecular and cellular analysis of human histamine receptor subtypes. Trends in Pharmacological Sciences, 34(1), 33–58.
https://doi.org/10.1016/j.tips.2012.11.001
Moreno, E., Hoffmann, H., González-Sepúlveda, M., Navarro, G., Casadó, V., Cortés, A., … & Ferré, S. (2011). Dopamine D1-histamine H3 receptor heteromers provide a selective link to MAPK signaling in GABAergic neurons of the direct striatal pathway. Journal of Biological Chemistry, 286(7), 5846–5854.
https://doi.org/10.1074/jbc.M110.161489
Rodríguez-Ruiz, M., Moreno, E., Moreno-Delgado, D., Navarro, G., Mallol, J., Cortés, A., … & Canela, E. I. (2017). Heteroreceptor complexes formed by dopamine D1, histamine H3, and NMDA glutamate receptors as targets to prevent neuronal death in Alzheimer’s disease. Molecular Neurobiology, 54(6), 4537–4550.
https://doi.org/10.1007/s12035-016-9995-y
Spaethling, J. M., Piel, D., Dueck, H., Buckley, P. T., Morris, J. F., Fisher, S. A., … & Eberwine, J. (2014). Serotonergic neuron regulation informed by in vivo single-cell transcriptomics. FASEB Journal, 28(2), 771–780.
https://doi.org/10.1096/fj.13-240267
Qian, H., Shu, C., Xiao, L., & Wang, G. (2022). Histamine and histamine receptors: Roles in major depressive disorder. Frontiers in Psychiatry, 13, 825591.
https://doi.org/10.3389/fpsyt.2022.825591
Dong, H., Zhang, W., Zeng, X., Hu, G., Zhang, H., He, S., & Zhang, S. (2014). Histamine induces upregulated expression of histamine receptors and increases release of inflammatory mediators from microglia. Molecular Neurobiology, 49(3), 1487–1500.
https://doi.org/10.1007/s12035-014-8697-6
Kumar, A., Dogra, S., Sona, C., Umrao, D., Rashid, M., Singh, S. K., & Malik, F. (2019). Chronic histamine 3 receptor antagonism alleviates depression-like conditions in mice via modulation of brain-derived neurotrophic factor and hypothalamus–pituitary–adrenal axis. Psychoneuroendocrinology, 101, 128–137.