HDAC Inhibitors: From "Pan-Inhibition" to Precision Therapy – A New Key to Overcoming Cancer Drug Resistance
Abstract
Histone deacetylase (HDAC) inhibitors have emerged as pivotal agents in epigenetic therapy, primarily used in hematologic malignancies due to the toxicity profiles of early-generation pan-inhibitors. Recent advances in isoform-selective HDAC inhibitors, PROTAC degraders, and innovative combination strategies have redefined the therapeutic landscape, especially in solid tumors. This article reviews the clinical evolution, mechanistic insights, biomarker challenges, and emerging therapeutic directions of HDAC inhibitors. With growing evidence supporting combination therapies and precision targeting, HDAC inhibitors are poised for a transformative resurgence in oncology and beyond.
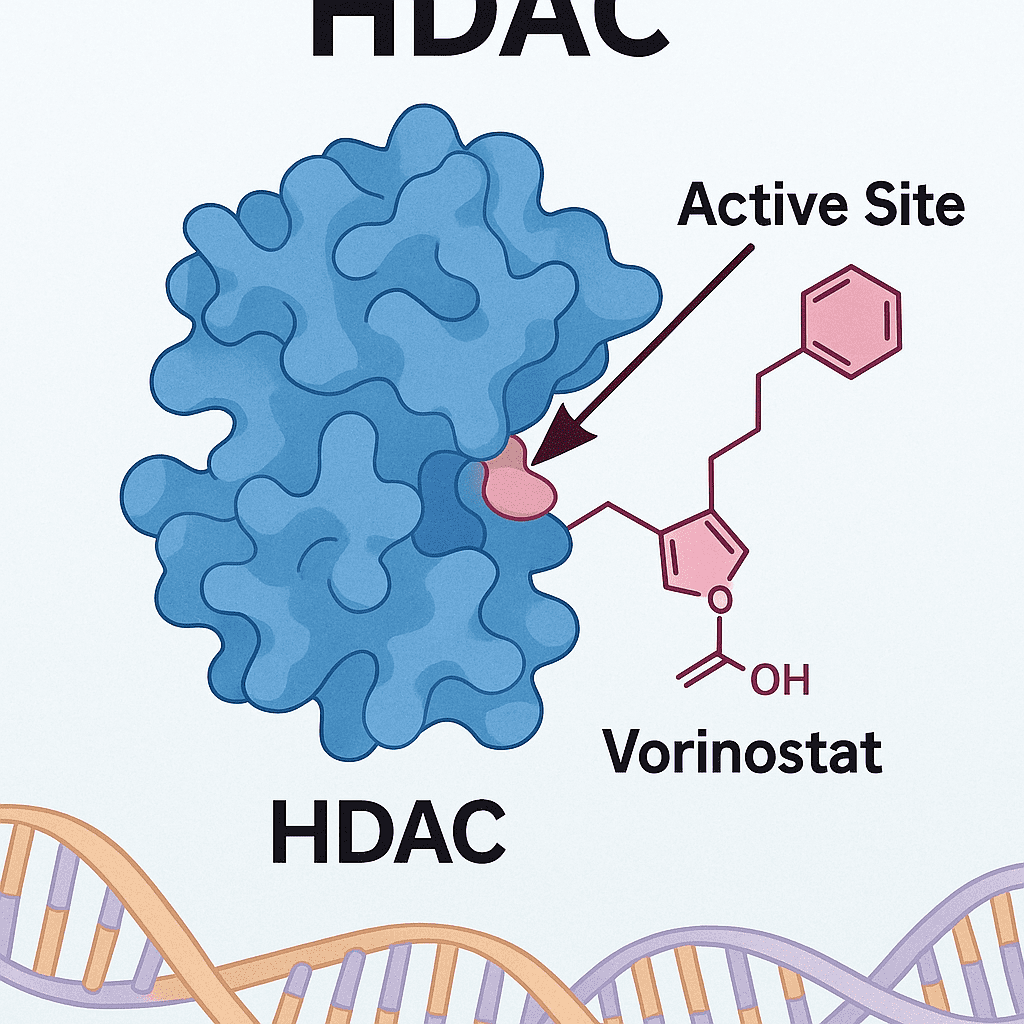
Histone deacetylase (HDAC) inhibitors regulate chromatin structure and gene expression, establishing themselves as pivotal targets in epigenetic therapy. However, traditional pan-HDAC inhibitors (e.g., vorinostat, panobinostat) have long been constrained to hematologic malignancies due to off-target effects across multiple HDAC isoforms, causing severe toxicities such as bone marrow suppression and cardiotoxicity. In recent years, the rise of isoform-selective inhibitors and novel technologies such as PROTAC-based degraders is driving a revolutionary transition—from broad-spectrum inhibition to precision oncology.
I. Clinical Development Landscape: Breakthroughs and Bottlenecks
1. Approved Drugs: Hematologic Cancers Still Dominate, Solid Tumors Struggle to Break Through
To date, five HDAC inhibitors have received global regulatory approval: vorinostat, romidepsin, belinostat, panobinostat, and chidamide. These are mainly indicated for cutaneous and peripheral T-cell lymphomas (CTCL/PTCL) and multiple myeloma (MM). Chidamide (developed by Chipscreen Biosciences) became China’s first original HDAC inhibitor and notably the first to succeed in a solid tumor indication—approved in 2019 for HR+ breast cancer in combination with exemestane.
While hematologic malignancies remain the foundation of HDAC inhibitor applications, their growth potential is limited. The current competition focuses on optimizing combination regimens and minimizing toxicity. Single-agent pan-HDAC inhibitors typically show low overall response rates (ORR <30%) and high rates of ≥Grade 3 hematologic toxicities (40%-50%).
Key Competitive Areas:
Multiple Myeloma (MM): The panobinostat + bortezomib + dexamethasone regimen is a standard of care. Selective HDAC6 inhibitors (e.g., Ricolinostat/ACY-241) are emerging as safer alternatives, aiming for enhanced tolerability and efficacy.
T-cell Lymphomas (TCL): Romidepsin, vorinostat, and chidamide form the current backbone of treatment. New entrants must demonstrate clear superiority in efficacy or safety.
2. Competitive Landscape of Clinical-Stage HDAC Inhibitors
The field is shifting from a saturated market of first-generation pan-inhibitors to a new wave focused on isoform selectivity, differentiated combination strategies, and cutting-edge technologies (e.g., PROTAC, allosteric inhibition). Multinational giants (Novartis, Celgene, Merck) dominate hematologic indications through acquisitions and collaborations. Meanwhile, Chinese and U.S. biotech companies are intensively pursuing solid tumor breakthroughs.
As of August 2025, there are 9 HDAC inhibitors in clinical development in China alone, with a strong focus on solid tumors (e.g., lung and breast cancers) and isoform-selective agents (e.g., HDAC6, HDAC8).
Solid tumors are the “Holy Grail.” Success will depend on identifying the right combination strategies and predictive biomarkers.
Hot Areas and Key Players:
HR+ Breast Cancer: Chidamide plus exemestane is already approved in China, securing first-mover advantage. Syndax’s entinostat is a key global competitor, with Phase III data from the E2112 trial under close watch.
PD-1/L1 Combinations: One of the most intensively pursued areas. Chipscreen is a frontrunner with promising data, while numerous other companies are exploring HDACi-immunotherapy combos across cancers like lung, liver, and gastric cancers.
Other solid tumors (lung, GI, neuroendocrine, etc.) remain in early stages of investigation—offering high potential but also considerable risk.
Emerging technologies like PROTAC-based HDAC degraders (e.g., KT-474 by Kymera Therapeutics) and dual-target inhibitors (e.g., HDAC/PD-1 combinations) are pushing the frontier.
II. Core Challenges: Toxicity, Resistance, and Biomarker Discovery
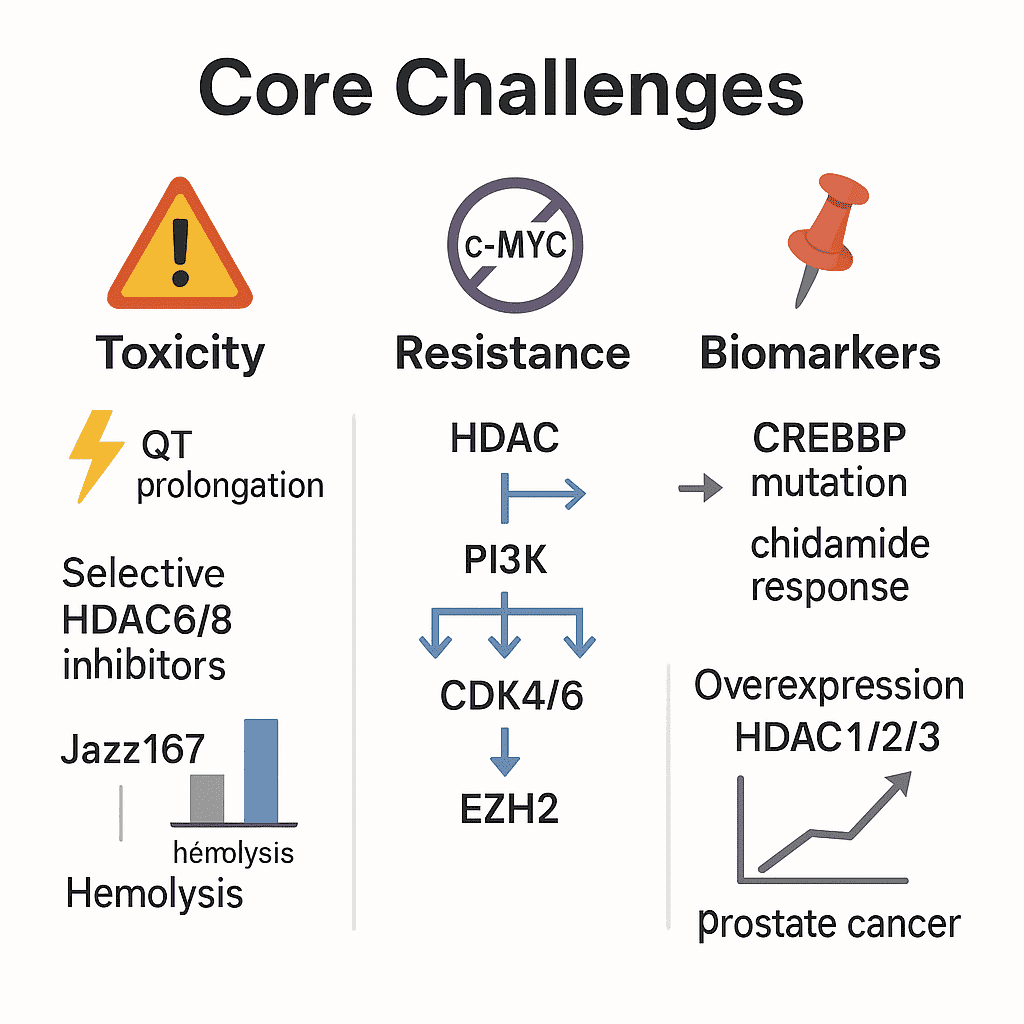
Toxicity Management
Cardiotoxicity, particularly QT prolongation, is mostly associated with HDAC2 inhibition. Selective HDAC6/8 inhibitors are being engineered to avoid this via precision targeting. Novel metal-based HDAC inhibitors (e.g., Jazz167) demonstrate reduced hemolytic activity (10% vs. 100% for cisplatin) in AR-null prostate cancer models, suggesting improved safety profiles.
Resistance Mechanisms
HDAC inhibitor monotherapy often activates compensatory pathways such as c-MYC. Combination therapies with PI3K or CDK4/6 inhibitors can help overcome resistance.
Epigenetic synergy: HDAC inhibitors combined with EZH2 inhibitors have shown enhanced DNA damage response in ovarian cancer models.
Biomarker Gaps
So far, only CREBBP mutations have been correlated with response to chidamide. Overexpression of HDAC1/2/3 has been linked to higher Gleason scores in prostate cancer, suggesting potential as predictive markers.
III. Latest Clinical Advances and Research Milestones
1. Chidamide’s New Indications
In April 2024, China’s NMPA approved chidamide in combination with R-CHOP for newly diagnosed MYC/BCL2-positive diffuse large B-cell lymphoma (DLBCL), based on interim data from the DEB trial (NCT04231448). Results showed improved complete remission rates and event-free survival (EFS) compared to standard R-CHOP.
At the 2025 ASCO Annual Meeting, results from the SCENT 2.0 trial were presented: chidamide combined with PD-1 inhibitors significantly remodeled the tumor microenvironment in treatment-naïve NK/T-cell lymphoma, demonstrating clinical efficacy and overcoming immune resistance.
2. CAPability-01 Trial Results
Published in Nature Medicine in March 2024, the CAPability-01 study led by Sun Yat-sen University showed that the combination of chidamide + sintilimab + bevacizumab achieved a 44.0% ORR and median PFS of 7.3 months in heavily pretreated MSS/pMMR metastatic colorectal cancer patients—a population historically resistant to immunotherapy.
3. Entinostat Approved in China
In April 2024, entinostat was approved by China’s NMPA for HR+/HER2- advanced or metastatic breast cancer patients who relapsed or progressed on endocrine therapy. Phase III data showed that entinostat significantly prolonged PFS (6.32 vs. 3.72 months), with a 24% reduction in progression risk and a median OS of 38.39 months (17% reduction in death risk).
IV. Future Directions: Precision, Combination, and Resistance Overcome
1. Precision Development of Isoform-Selective Inhibitors
HDAC6 Inhibitors: HDAC6-knockout mice are viable, and selective targeting greatly reduces hematologic toxicity (platelet reduction <20% vs. 40–50% with pan-inhibitors). Ricolinostat combined with bortezomib shows 55–71% ORR in MM with a favorable safety profile. Non-hydroxamate scaffolds (e.g., chromone derivatives) demonstrate IC50 as low as 70 nM, avoiding the metal-chelation toxicity of traditional hydroxamic acids.
HDAC1/2/3 Selective Inhibitors in Solid Tumors: Entinostat combined with endocrine therapy extended mPFS from 3.8 to 7.4 months (ACE study), but predictive biomarker identification remains a bottleneck.
Resistance Targets: HDAC3 inhibition upregulates CD47, promoting M2 macrophage polarization. Blocking the CD47-SIRPα axis can reverse this.
2. Innovation in Combination Therapy
Immunotherapy Synergy: HDAC inhibitors upregulate PD-L1 and tumor antigens, enhancing responsiveness to PD-1/CTLA-4 blockade. Chidamide + sintilimab yielded ORRs of 58.3% in ENKTL and 71% in melanoma.
Targeted Synergies:
HDAC + EZH2: Strong synergy in DNA repair inhibition, especially in ovarian cancer models.
HDAC + Radiotherapy: Downregulation of DNA repair proteins like Rad51 sensitizes tumors to radiation, potentially reducing dosage by 30–50%.
3. Resistance Mechanism Insights
CD47-SIRPα Axis: Upregulated by HDACi, this pathway drives immune evasion; its inhibition restores antitumor immunity.
TSPYL5 as a Biomarker: High expression confers resistance to HDAC inhibitors; low expression doubles response rate.
4. Non-Oncological Expansions
Neurodegenerative Disorders: HDAC3 modulates neuronal plasticity and may improve cognition in Alzheimer’s disease models.
HIV Latency Reversal: Vorinostat is under Phase II clinical evaluation for reversing HIV latency when combined with antiretroviral therapy.
Conclusion: The “Second Spring” of HDAC Inhibitors
From hematologic malignancies to solid tumors, from pan-inhibitors to PROTAC degraders, HDAC inhibitors are being reborn through technological innovation and refined clinical strategies. Over the next five years, breakthroughs in biomarker discovery and next-gen inhibitors may position HDACs as pivotal in overcoming tumor resistance.
Winning factors for the future:
Clinical success of combination regimens: The first player to achieve Phase III validation in a major solid tumor may capture significant market share.
Biomarker-guided precision: Patient stratification based on validated biomarkers will be key to maximizing efficacy and minimizing risk.
Clinical validation of next-gen technologies: Early PROTAC and allosteric inhibitor data will define the next competitive cycle.
References
Cheng B, Pan W, Xiao Y, Ding Z, Zhou Y, Fei X, Liu J, Su Z, Peng X, Chen J. HDAC-targeting epigenetic modulators for cancer immunotherapy. Eur J Med Chem. 2024 Feb 5;265:116129. doi: 10.1016/j.ejmech.2024.116129. Epub 2024 Jan 6. PMID: 38211468.
https://www.sciencedirect.com/science/article/abs/pii/S0223523424000096
Ramaiah MJ, Tangutur AD, Manyam RR. Epigenetic modulation and understanding of HDAC inhibitors in cancer therapy. Life Sci. 2021 Jul 15;277:119504. doi: 10.1016/j.lfs.2021.119504. Epub 2021 Apr 16. PMID: 33872660.
https://www.sciencedirect.com/science/article/abs/pii/S0024320521004896
Chen S, Zheng Y, Liang B, Yin Y, Yao J, Wang Q, Liu Y, Neamati N. The application of PROTAC in HDAC. Eur J Med Chem. 2023 Nov 15;260:115746. doi: 10.1016/j.ejmech.2023.115746. Epub 2023 Aug 19. PMID: 37607440.
https://www.sciencedirect.com/science/article/abs/pii/S0223523423007134
Ling R, Wang J, Fang Y, Yu Y, Su Y, Sun W, Li X, Tang X. HDAC-an important target for improving tumor radiotherapy resistance. Front Oncol. 2023 Jul 12;13:1193637. doi: 10.3389/fonc.2023.1193637. PMID: 37503317; PMCID: PMC10368992.
https://pmc.ncbi.nlm.nih.gov/articles/PMC10368992/
Shanmugam G, Rakshit S, Sarkar K. HDAC inhibitors: Targets for tumor therapy, immune modulation and lung diseases. Transl Oncol. 2022 Feb;16:101312. doi: 10.1016/j.tranon.2021.101312. Epub 2021 Dec 16. PMID: 34922087; PMCID: PMC8688863.
https://www.sciencedirect.com/science/article/pii/S193652332100303X
Huang Z, Zeng L, Cheng B, Li D. Overview of class I HDAC modulators: Inhibitors and degraders. Eur J Med Chem. 2024 Oct 5;276:116696. doi: 10.1016/j.ejmech.2024.116696. Epub 2024 Jul 30. PMID: 39094429.
https://www.sciencedirect.com/science/article/abs/pii/S0223523424005762