Gepotidacin: A Game-Changer in the Fight Against Antibiotic Resistance
Abstract
Gepotidacin, branded as Blujepa, is a groundbreaking antibiotic approved by the FDA in 2025 for the treatment of uncomplicated urinary tract infections and gonorrhea. As the first in a new class of topoisomerase inhibitors, Gepotidacin offers a unique mechanism of action that addresses rising antimicrobial resistance. Clinical trials demonstrated strong efficacy and safety, positioning the drug as a valuable oral alternative to traditional treatments. Its approval signals a critical shift in antibiotic innovation, offering renewed hope for global public health and drug-resistant infection management.
Introduction: Why Gepotidacin Matters in 2025
In 2025, the global fight against antibiotic resistance reached a critical milestone with the FDA approval of Gepotidacin (brand name Blujepa). This novel antibiotic marks the first of a new drug class to be approved in over two decades, offering hope in an era where traditional antibiotics are losing their effectiveness against resistant bacteria.
The World Health Organization has repeatedly emphasized the urgent need for innovative antimicrobial agents, especially those effective against urinary tract infections (UTIs) and sexually transmitted infections (STIs) like gonorrhea, which have shown alarming resistance to current treatments. Gepotidacin was specifically developed to address this crisis.
What sets Gepotidacin apart is its unique mechanism of action. Unlike fluoroquinolones and macrolides, which have faced widespread resistance, Gepotidacin targets bacterial DNA gyrase and topoisomerase IV in a novel way, reducing the likelihood of cross-resistance. This makes it a strong candidate for multi-drug resistant infections, especially those caused by Escherichia coli and Neisseria gonorrhoeae.
Its approval came after promising results from large Phase III clinical trials—EAGLE-1 and EAGLE-2—that demonstrated its non-inferiority compared to existing treatments, with a favorable safety profile. As a first-in-class, oral antibiotic, Gepotidacin may significantly reduce reliance on injectable treatments, providing more accessible options for patients.
In a landscape threatened by antibiotic failure, Gepotidacin represents both scientific innovation and strategic urgency—a symbol of what’s possible when pharmaceutical advancement aligns with public health needs.
What is Gepotidacin? Understanding the Science
Gepotidacin (brand name: Blujepa) is a first-in-class antibiotic in a novel class of drugs known as triazaacenaphthylene topoisomerase inhibitors. Unlike traditional antibiotics such as fluoroquinolones, beta-lactams, or macrolides, Gepotidacin targets two essential bacterial enzymes—DNA gyrase and topoisomerase IV—but binds at a unique site not shared with existing drugs. This means it retains activity even against bacterial strains that have become resistant to other antibiotic classes.
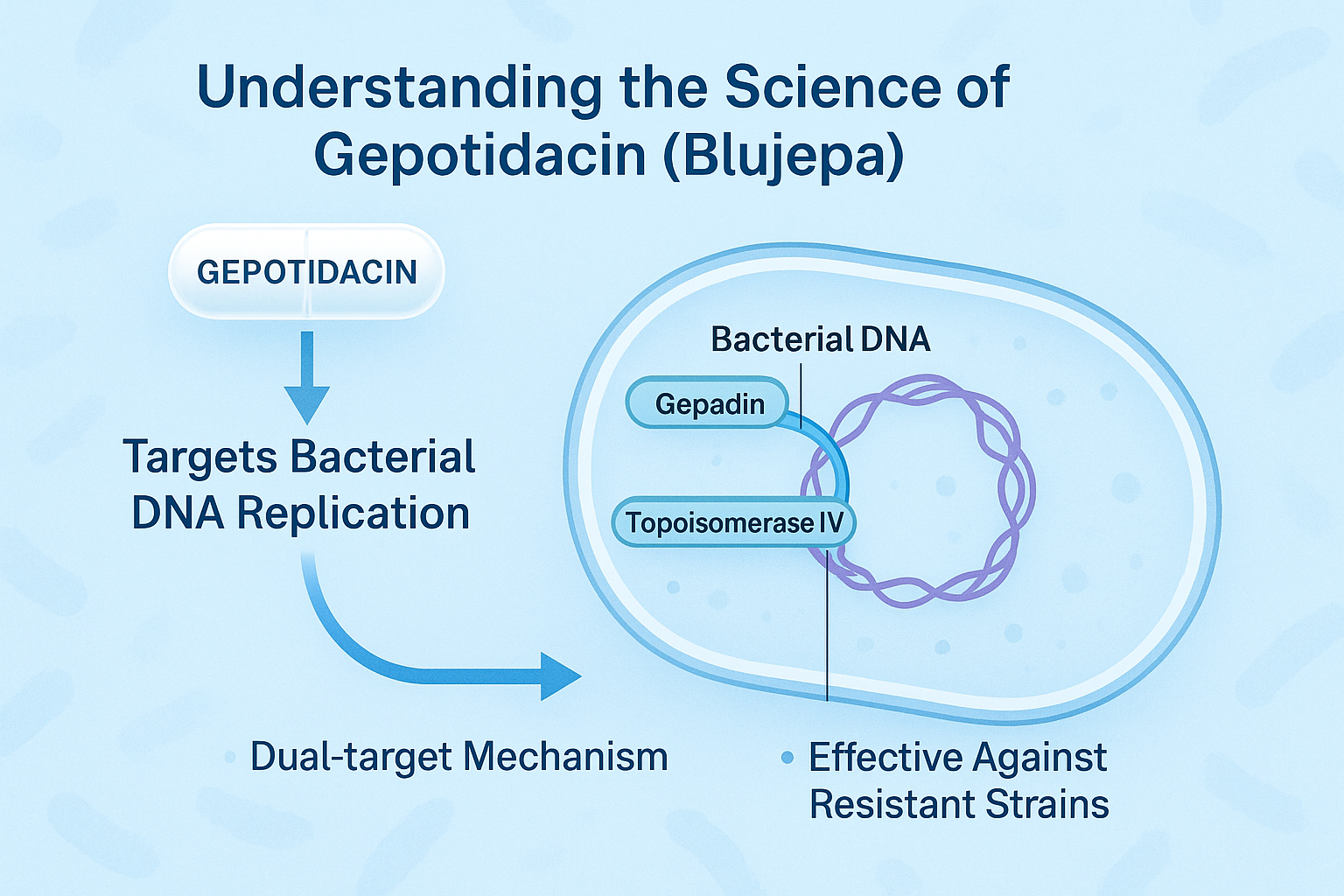
The innovation behind Gepotidacin lies in its dual-target mechanism, which disrupts bacterial DNA replication and repair. By interfering with these processes, it effectively kills or halts the growth of pathogens like Escherichia coli and Neisseria gonorrhoeae—two of the most common causes of urinary tract infections (UTIs) and gonorrhea, respectively.
Unlike many existing antibiotics that require intravenous administration, Gepotidacin is administered orally, making it easier for outpatient treatment and global distribution. Its pharmacokinetics show effective tissue penetration and minimal risk for severe adverse reactions, especially QT prolongation or C. difficile infection, common concerns with other classes.
Importantly, Gepotidacin’s unique binding action means that cross-resistance with other antibiotics is minimal, and resistance development is expected to be slower. This gives it a promising role in antimicrobial stewardship efforts globally.
With bacterial resistance on the rise and the antibiotic pipeline historically stagnant, Gepotidacin emerges as a critical tool in a new generation of precision antibiotics, balancing efficacy, safety, and strategic resistance mitigation.
FDA Approval & Clinical Trials: The Road to Gepotidacin
In March 2025, the U.S. Food and Drug Administration (FDA) approved Gepotidacin (Blujepa) as a first-in-class oral antibiotic for the treatment of uncomplicated urinary tract infections (uUTIs) and urethral and rectal gonorrhea. This marked a pivotal moment in antimicrobial drug development, making Gepotidacin the first new oral antibiotic class approved in over 20 years.
The approval was based on compelling results from two Phase III clinical trials—EAGLE-1 and EAGLE-2—which demonstrated that Gepotidacin was non-inferior to standard-of-care treatments such as nitrofurantoin and ceftriaxone + azithromycin.
EAGLE-1, a randomized, multicenter trial, evaluated Gepotidacin in patients with urogenital gonorrhea. It showed cure rates comparable to ceftriaxone plus azithromycin, with fewer gastrointestinal side effects and no injectable dosing required.
EAGLE-2 focused on uncomplicated UTIs primarily caused by E. coli. The trial included over 3,000 patients globally and found that a 5-day oral course of Gepotidacin was as effective as a 5-day course of nitrofurantoin, with similar safety outcomes.
The trials also emphasized Gepotidacin’s tolerability, with mild-to-moderate side effects like headache and diarrhea being the most common. No cases of serious drug-induced liver injury or QT prolongation were reported.
These successful trials helped validate Gepotidacin’s potential not only as a treatment option for common infections, but also as a strategic weapon against rising antimicrobial resistance, especially in outpatient settings where oral therapies are preferred.
Use Cases: UTIs, Gonorrhea, and Beyond
Gepotidacin (Blujepa) is currently approved for the treatment of two widespread and clinically important infections: uncomplicated urinary tract infections (uUTIs) and uncomplicated urogenital gonorrhea. These conditions affect millions globally each year and are increasingly resistant to standard treatments.
In urinary tract infections, Gepotidacin targets Escherichia coli and Staphylococcus saprophyticus, the two most common causative bacteria. Thanks to its oral formulation and 5-day dosing regimen, it’s especially valuable in outpatient settings, helping reduce hospitalization and the need for IV antibiotics. Clinical trials show it performs as well as nitrofurantoin, with a favorable safety profile and minimal side effects.
In cases of gonorrhea, especially with strains resistant to ceftriaxone and azithromycin, Gepotidacin offers a breakthrough. It is the first oral treatment shown to be effective without needing a combination therapy or intramuscular injection. This is crucial for treating infections in low-resource settings or for patients with injection intolerance or needle aversion.
Beyond these two indications, Gepotidacin is being investigated for broader antimicrobial applications. Research is underway to assess its efficacy against resistant strains of Streptococcus pneumoniae, Mycoplasma genitalium, and other sexually transmitted infections. Its novel mechanism makes it a promising candidate for future uses in combating multi-drug resistant pathogens.
With oral delivery, broad-spectrum potential, and effectiveness against resistant organisms, Gepotidacin is positioned to play a key role in the next generation of outpatient antibiotics, especially as resistance to conventional agents continues to rise.
Future Outlook & Why Gepotidacin Matters
The approval of Gepotidacin in 2025 is more than just another pharmaceutical milestone — it’s a turning point in the global response to antimicrobial resistance (AMR). As resistant strains of E. coli and Neisseria gonorrhoeae continue to spread worldwide, the arrival of a first-in-class oral antibiotic offers a rare but urgent breakthrough.
From a public health standpoint, Gepotidacin represents a strategic step toward antimicrobial stewardship. Unlike traditional antibiotics that often fail due to overuse or poor targeting, Gepotidacin is specifically engineered to reduce the development of resistance through its novel dual-binding mechanism. This innovation could help preserve its efficacy longer than past drug classes.
Economically, the drug also signals a renewed investment in antibiotic R&D—a field that had stagnated for decades due to low commercial incentive. Its approval may encourage pharmaceutical companies to resume development of other novel antimicrobials, especially those targeting community-acquired infections.
Looking forward, Gepotidacin may also be evaluated for expanded indications, including multi-drug resistant respiratory tract infections, Mycoplasma genitalium, and even prophylactic use in high-risk patients. Its oral dosing, safety profile, and resistance coverage make it a strong candidate for future global treatment protocols, especially in low- and middle-income countries where injectable antibiotics are harder to deploy.
In summary, Gepotidacin matters because it signals hope: hope for clinicians seeking effective treatments, for patients suffering from recurring infections, and for a world increasingly threatened by antibiotic resistance. It’s not just a new drug—it’s a new direction.
References
Keam, S. J. (2025). Gepotidacin: First approval. Drugs.
https://link.springer.com/article/10.1007/s40265-025-02214-9
Durham, S. H., & Chahine, E. B. (2025). Gepotidacin: A novel antibiotic for uncomplicated urinary tract infections. Annals of Pharmacotherapy.
https://journals.sagepub.com/doi/abs/10.1177/10600280251343682
Bax, B. D., et al. (2021). Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature, 590(7845), 112–117.