From Signals to Division: How Growth Factors Drive the Cell Cycle and Shape Cancer Therapy
Abstract
Growth factors (GFs) are essential regulators of cell proliferation, acting through receptor tyrosine kinases (RTKs) to activate intracellular signaling pathways that control progression through the cell cycle. While their influence on G1 phase entry is well-established, recent studies have uncovered broader roles for growth factor-induced signaling, including impacts on DNA replication, checkpoint control, and mitosis. Central to these processes are the Ras/Erk and PI3K/Akt pathways, which regulate key cell cycle proteins such as cyclin D1, p21^Cip1, and p27^Kip1. This blog post synthesizes insights from a comprehensive review by Wang (2021), highlighting the dynamic interplay between mitogenic signaling and the core cell cycle machinery. Understanding these pathways not only deepens our grasp of cell biology but also offers valuable implications for cancer research and the development of targeted therapies.
Introduction: Why Growth Factor Signaling Matters in Cell Biology
In the complex choreography of life, the cell cycle is the fundamental process that enables cells to grow, replicate, and divide. From embryonic development to tissue regeneration and immune responses, proper control of the cell cycle ensures that cells function harmoniously within the body. When this process goes awry, the consequences can be serious—most notably, uncontrolled cell proliferation seen in cancer.
At the heart of cell cycle regulation lies a network of signaling molecules known as growth factors (GFs). These extracellular proteins bind to receptor tyrosine kinases (RTKs) on the cell surface, initiating signaling cascades that influence whether a cell should divide, differentiate, or remain quiescent. Among the many pathways involved, the Ras/Erk and PI3K/Akt pathways are two of the most studied and are known to play pivotal roles in cell cycle progression, particularly at the G1 phase.
Growth factor signaling acts as the external “green light” that integrates environmental conditions—like nutrient availability, stress, or damage—with the internal machinery that drives the cell forward. Understanding how these pathways regulate each phase of the cell cycle isn’t just of academic interest. It’s central to advancing cancer research, regenerative medicine, and targeted therapy development. When these signals are mutated, amplified, or deregulated, they can bypass crucial cell cycle checkpoints, enabling tumor cells to grow unchecked.
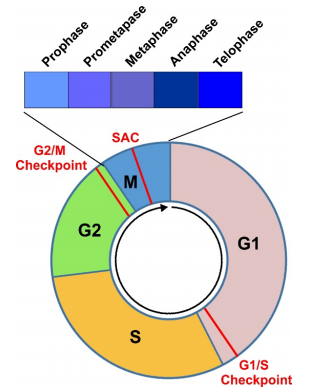
Figure 1. Diagram to illustrate a complete cell cycle progression through four cell cycle phases(G1, S, G2, and M) and three major checkpoints (G1/S, G2/M, and SAC).
In this blog series, we’ll explore a landmark review published in Cells, which synthesizes decades of research into how growth factor-induced signaling regulates the cell cycle. While G1 phase control is well-characterized, recent findings show that GFs may also impact S, G2, and M phases—challenging older models and opening new avenues for research and therapeutic intervention.
The Basics: Cell Cycle Phases and Checkpoints
The cell cycle is the highly regulated series of events that allows a single cell to replicate its DNA and divide into two daughter cells. It is essential for growth, tissue repair, and reproduction. At its core, the cell cycle is composed of four main stages: G1 (Gap 1), S (Synthesis), G2 (Gap 2), and M (Mitosis). These stages are collectively governed by complex molecular networks that ensure the cell divides only when it is safe and appropriate to do so.
The G1 phase is the first step after mitosis, where the cell grows and prepares the necessary building blocks for DNA replication. This is followed by the S phase, during which DNA replication occurs. The G2 phase provides a final checkpoint to ensure DNA replication has completed successfully and allows the cell to prepare for mitosis. The final step, the M phase, involves the physical division of the nucleus (mitosis) and the cell (cytokinesis).
To maintain order, the cycle includes three major checkpoints:
G1/S Checkpoint (also known as the restriction point): Determines whether the cell is ready to replicate DNA.
G2/M Checkpoint: Ensures DNA has been accurately replicated before the cell enters mitosis.
Spindle Assembly Checkpoint (SAC): Verifies that all chromosomes are properly attached to the mitotic spindle before the cell divides.
At the molecular level, cyclins and cyclin-dependent kinases (Cdks) act as the core regulators of phase transitions. Cyclins fluctuate in abundance throughout the cycle, binding and activating Cdks at precise stages. These Cdk–cyclin complexes phosphorylate target proteins to drive the cell forward. If errors are detected—such as DNA damage or incomplete replication—checkpoint mechanisms will halt progression to prevent genomic instability.
Understanding this system is essential not only for cell biology but also for cancer research, where mutations in checkpoint proteins often lead to uncontrolled proliferation and tumor development.
Ras/Erk and PI3K/Akt: The Power Duo Behind G1 Phase Progression
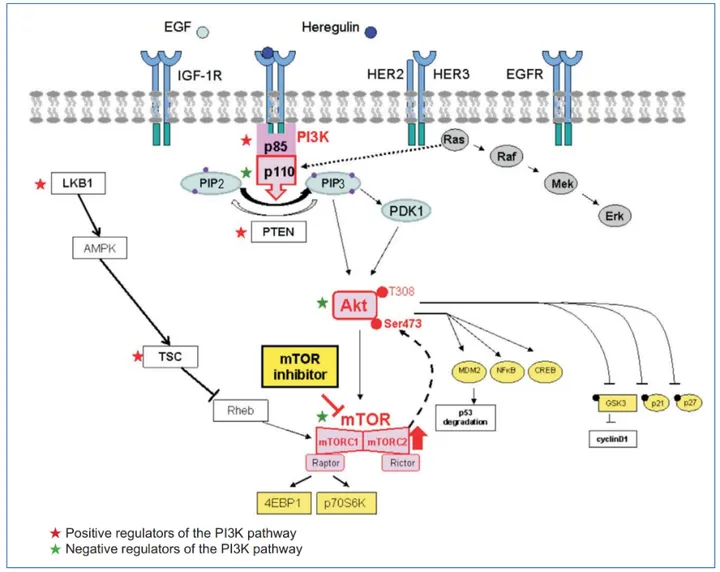
One of the most critical transitions in the cell cycle occurs in the G1 phase, where cells commit to DNA replication and division. This decision point—known as the G1/S checkpoint or restriction point—is where growth factor (GF) signaling takes center stage. Among the many pathways activated by growth factors, two stand out as key regulators of cell cycle progression: the Ras/Erk pathway and the PI3K/Akt pathway.
Upon growth factor binding, receptor tyrosine kinases (RTKs) become activated, triggering downstream signaling cascades. The Ras/Erk pathway begins with activation of Ras, which in turn activates Raf, MEK, and eventually Erk (extracellular signal-regulated kinase). Activated Erk translocates to the nucleus and induces the expression of cyclin D1, a key regulator that pairs with Cdk4/6 to phosphorylate the retinoblastoma (Rb) protein. This releases the transcription factor E2F, enabling the transcription of genes needed for DNA synthesis.
At the same time, growth factors stimulate the PI3K/Akt pathway, which promotes cell survival and proliferation. Akt, once activated, enhances cyclin D1 stability, inhibits Cdk inhibitors like p27^Kip1, and promotes cellular growth through activation of mTORC1 and increased protein synthesis. Akt also stabilizes p21^Cip1, which, despite being known as a Cdk inhibitor, paradoxically supports Cdk4 activity under certain conditions.
Together, Ras/Erk and PI3K/Akt signaling serve as a dual engine driving the cell past the G1/S checkpoint. Their crosstalk ensures precise control—while either pathway alone contributes to G1 progression, full commitment to DNA replication typically requires both. This integrated regulation is not only critical for normal physiology but is also hijacked in many cancers where these pathways become hyperactivated.
Understanding this “power duo” has helped shape targeted therapies, particularly MEK inhibitors and PI3K inhibitors, which aim to reassert control over unregulated proliferation in tumors.
Beyond G1: Emerging Roles of Growth Factors in S, G2, and M Phases
For years, it was widely accepted that growth factor (GF) signaling primarily governs the early G1 phase of the cell cycle, guiding cells toward the decision to divide. However, emerging research—including insights from Wang (2021)—suggests that growth factors and their downstream signaling pathways continue to play significant roles well beyond G1, extending their influence into the S, G2, and M phases.
During the S phase, when DNA replication occurs, both the PI3K/Akt and Ras/Erk pathways contribute to maintaining genome stability. Akt signaling promotes the activation of proteins involved in DNA replication and facilitates centrosome duplication, which is crucial for later stages of cell division. At the same time, ERK signaling helps coordinate replication origin firing and has been implicated in the modulation of histone gene expression, ensuring the newly synthesized DNA is properly packaged.
In the G2 phase, cells assess DNA integrity before committing to mitosis. Here, PI3K/Akt activity supports the DNA damage response, in part by regulating Chk1, a key checkpoint kinase. While Erk signaling is not as dominant in G2, its overactivation can delay progression, possibly by affecting proteins involved in chromatin condensation and repair.
Contrary to earlier assumptions that mitogenic signaling shuts down during mitosis, new evidence shows EGFR remains active during the M phase. Notably, EGFR preferentially signals through Akt2, rather than Akt1, in mitotic cells. Meanwhile, Ras fails to activate Erk during mitosis, indicating a pathway shift that might help maintain mitotic fidelity. Experimental inhibition of EGFR in this phase leads to mitotic delays, underscoring the pathway’s regulatory importance even in the final stage of cell division.
These discoveries challenge traditional models and open new avenues for targeted interventions. By understanding how GF signaling operates across all phases of the cycle, researchers can develop more precise therapies that exploit cancer cells’ dependency on continuous mitogenic input.
Conclusion: What This Means for Cancer Research and Drug Development
The tight regulation of the cell cycle is fundamental to healthy cellular function. When control over this process is lost, the consequences can be dire—most notably, cancer, where cells divide uncontrollably and evade normal growth constraints. As we’ve seen throughout this discussion, growth factor (GF)-induced signaling, particularly through the Ras/Erk and PI3K/Akt pathways, is not just important for initiating the cell cycle, but influences progression through multiple phases. This makes it a critical area of focus for both basic research and therapeutic innovation.
In cancer cells, growth factor pathways are frequently deregulated. Mutations in RTKs, constitutive activation of Ras, amplification of PI3K, or loss of PTEN (a negative regulator of PI3K) can result in persistent proliferative signaling. These alterations bypass checkpoint controls and decouple cell division from normal growth signals. As a result, understanding the nuances of how these pathways interact with cell cycle regulators is essential for designing targeted cancer therapies.
Over the past two decades, this knowledge has translated into the development of small molecule inhibitors and monoclonal antibodies that target RTKs (e.g., EGFR, HER2), Raf kinases, MEK, PI3K, and Akt. Despite these advances, drug resistance remains a major challenge. Cancer cells can often reroute signaling through alternative pathways or acquire new mutations, underscoring the need for deeper understanding and combination therapy strategies.
Furthermore, insights into growth factor signaling beyond G1—into S, G2, and M phases—are revealing new vulnerabilities. For instance, the discovery that EGFR signaling persists during mitosis opens the door for phase-specific interventions, potentially improving the timing and effectiveness of treatments.
As we move forward, integrating systems biology, single-cell analysis, and computational modeling with traditional molecular biology will be essential. These tools can help map the dynamic and context-dependent nature of GF signaling, ultimately leading to more precise, adaptive, and durable cancer therapies.
References
Massagué, J. (2004). G1 cell-cycle control and cancer. Nature, 432(7015), 298–306.
https://doi.org/10.1038/nature03094
Pardee, A. B. (1974). A restriction point for control of normal animal cell proliferation. Proceedings of the National Academy of Sciences, 71(4), 1286–1290.
https://doi.org/10.1073/pnas.71.4.1286
Wang, Z. (2021). Regulation of cell cycle progression by growth factor-induced cell signaling. Cells, 10(12), 3327.
https://doi.org/10.3390/cells10123327
Yarden, Y., & Sliwkowski, M. X. (2001). Untangling the ErbB signalling network. Nature Reviews Molecular Cell Biology, 2(2), 127–137.
https://doi.org/10.1038/35052073
Barnum, K. J., & O’Connell, M. J. (2014). Cell cycle regulation by checkpoints. Methods in Molecular Biology, 1170, 29–40.
https://doi.org/10.1007/978-1-4939-0888-2_2
Morgan, D. O. (1997). Cyclin-dependent kinases: Engines, clocks, and microprocessors. Annual Review of Cell and Developmental Biology, 13, 261–291.
https://doi.org/10.1146/annurev.cellbio.13.1.261
Satyanarayana, A., & Kaldis, P. (2009). Mammalian cell-cycle regulation: Several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene, 28(33), 2925–2939.
https://doi.org/10.1038/onc.2009.170
Meloche, S., & Pouysségur, J. (2007). The Erk1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene, 26(22), 3227–3239.
https://doi.org/10.1038/sj.onc.1210414
Chang, F., Lee, J. T., Navolanic, P. M., Steelman, L. S., & McCubrey, J. A. (2003). Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation. Leukemia, 17(3), 590–603.
https://doi.org/10.1038/sj.leu.2402824
Liu, Y., Zhang, Y., Schlüter, K. D., & Greber, B. (2016). EGFR signaling during mitosis: A new regulatory function in cell division. Cell Cycle, 15(19), 2648–2655.
https://doi.org/10.1080/15384101.2016.1211212
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144(5), 646–674.
https://doi.org/10.1016/j.cell.2011.02.013
Porta, C., Paglino, C., & Mosca, A. (2014). Targeting PI3K/Akt/mTOR signaling in cancer. Frontiers in Oncology, 4, 64.