From Cancer to Autoimmunity: How CAR-T Therapy Is Revolutionizing Chronic Disease Treatment
Abstract
CAR-T cell therapy, originally developed for treating blood cancers, is now showing transformative potential in managing autoimmune diseases such as lupus, multiple sclerosis, and rheumatoid arthritis. By genetically reprogramming T cells to target autoreactive immune cells, CAR-T offers deep immune modulation and long-term remission. Compared to traditional treatments, it provides greater precision, durability, and reduced need for chronic dosing. Clinical trials report promising outcomes with minimal side effects, while innovations like CAR-Tregs and off-the-shelf therapies are expanding its reach. Although cost and safety challenges remain, CAR-T therapy marks a paradigm shift in how we approach immune-related disorders, offering hope for a curative approach in autoimmunity.
Introduction: A New Frontier for CAR-T Cell Therapy
Chimeric Antigen Receptor T cell therapy—widely known as CAR-T cell therapy—has already transformed the landscape of cancer treatment. Originally developed to combat blood cancers such as leukemia and lymphoma, CAR-T therapy involves engineering a patient’s T cells to recognize and eliminate specific cell targets. This approach has yielded remarkable results, with many patients achieving complete remission after a single infusion.
Now, CAR-T therapy is venturing beyond oncology. Autoimmune diseases, which include chronic conditions like systemic lupus erythematosus (SLE), multiple sclerosis (MS), and rheumatoid arthritis (RA), are driven by an overactive immune system attacking the body’s own cells. Traditional treatments rely on broad-spectrum immunosuppressants and monoclonal antibodies, which often come with side effects and require ongoing administration.
Emerging research suggests that CAR-T therapy can offer a more precise and lasting solution. By targeting autoreactive B cells and T cells, CAR-T cells have demonstrated the potential to not only suppress disease activity but also to induce long-term remission. Clinical trials are now underway to explore CAR-T therapy for conditions like SLE, idiopathic inflammatory myositis, and even autoimmune diabetes.
This breakthrough marks a paradigm shift in how we approach immune-mediated disorders. Rather than simply managing symptoms, CAR-T cell therapy offers the possibility of reprogramming the immune system at its root. As the first wave of clinical data shows promising safety and efficacy, researchers and clinicians alike are optimistic about the role of CAR-T in the next generation of autoimmune disease therapies.
What Is CAR-T Cell Therapy?
CAR-T cell therapy is a groundbreaking form of immunotherapy that harnesses the body’s own immune system to fight disease. The process involves collecting a patient’s T cells (a type of white blood cell), genetically engineering them to express a chimeric antigen receptor (CAR), and reinfusing them into the patient. These engineered T cells can now identify and destroy cells that express specific surface markers.
Originally developed for hematologic cancers, CAR-T therapy has led to unprecedented remission rates in conditions such as acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Six CAR-T products have been approved by the FDA for cancer indications, with targets like CD19 and BCMA being central to their effectiveness.
What makes CAR-T therapy especially compelling is its “living drug” quality. Unlike traditional drugs, CAR-T cells can proliferate inside the body, offering sustained surveillance against disease recurrence. This long-term persistence provides a unique advantage over monoclonal antibodies, which often require repeated dosing and provide limited tissue penetration.
Recent advancements now explore its applications beyond cancer. For autoimmune diseases—conditions in which the immune system mistakenly attacks the body’s own tissues—CAR-T cells are being repurposed to eliminate autoreactive immune cells, restoring immune balance and potentially inducing remission.
This evolution from cancer immunotherapy to immune system reprogramming highlights CAR-T’s versatility and revolutionary potential.
Why Target Autoimmune Diseases?
Autoimmune diseases affect millions of people worldwide and are notoriously complex to treat. These conditions occur when the immune system mistakenly identifies healthy tissues as foreign invaders, leading to chronic inflammation and tissue damage. Common autoimmune diseases include rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS), and type 1 diabetes (T1DM).
The traditional management of autoimmune diseases relies on immunosuppressive drugs and biologic agents such as monoclonal antibodies. While effective in some cases, these treatments often require lifelong administration, increase the risk of infections, and fail to offer a cure.
CAR-T cell therapy introduces a paradigm shift. Rather than dampening the entire immune system, CAR-T cells can selectively target and eliminate the autoreactive B or T cells responsible for the disease. For example, CD19-targeting CAR-T cells have shown the ability to deplete pathological B cells that produce autoantibodies—key drivers in conditions like SLE and MS.
Moreover, CAR-Tregs (regulatory T cells engineered with CARs) can suppress immune attacks in a more balanced and controlled manner, maintaining immune homeostasis without global suppression. This makes them promising candidates for diseases where overactive T cell responses play a central role.
Given the precision and adaptability of CAR-T therapy, it holds the potential not just to manage, but to reset the immune system, leading to long-term remission. With multiple early-phase trials showing remarkable outcomes, CAR-T is emerging as a game-changer for autoimmunity.
Mechanisms – How CAR-T Cells Work in Autoimmunity
CAR-T cell therapy works by genetically reprogramming T cells to attack specific immune targets. In cancer, these targets are tumor-associated antigens. In autoimmune diseases, the targets are typically autoreactive B or T cells that mistakenly attack the body’s own tissues.
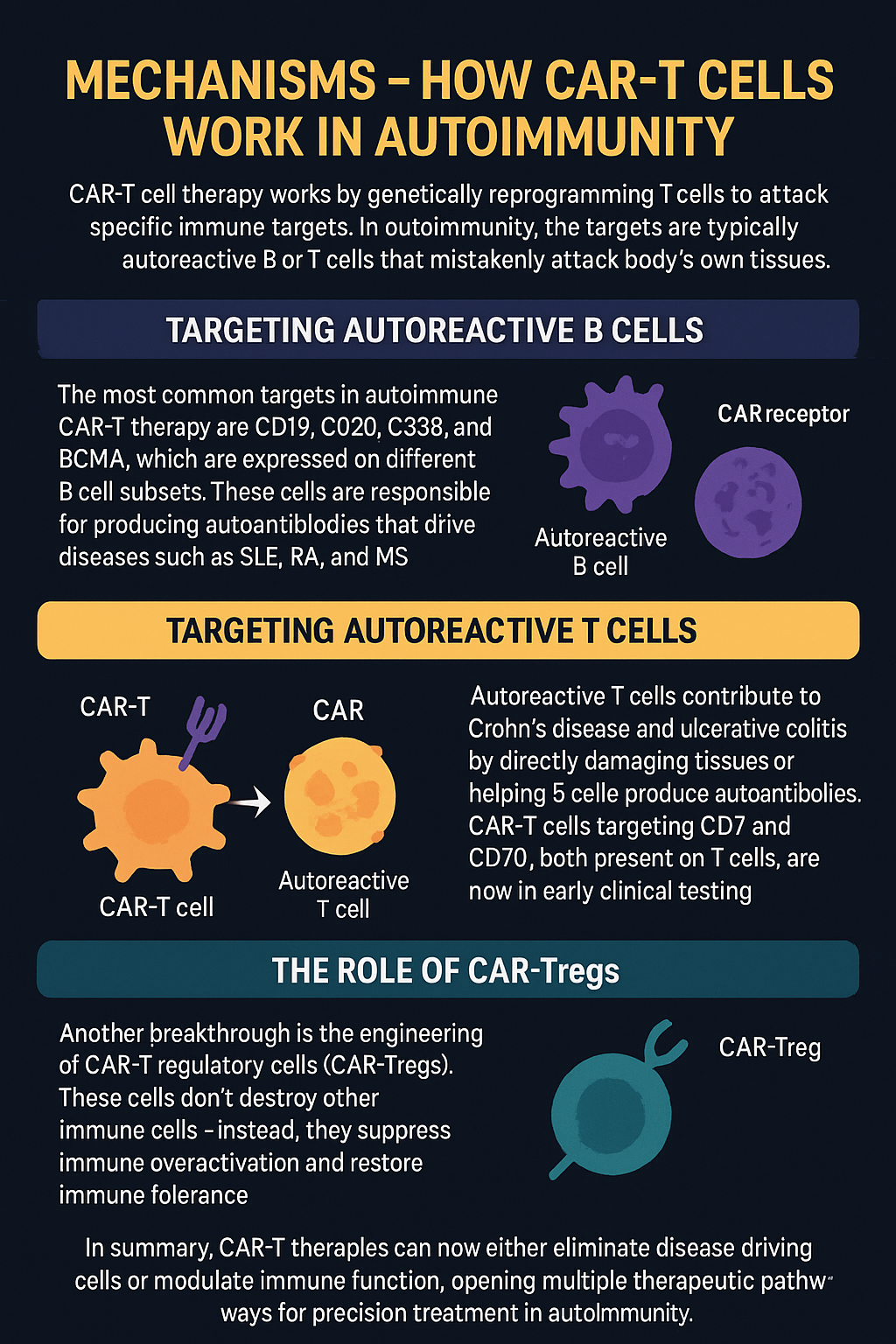
Targeting Autoreactive B Cells
The most common targets in autoimmune CAR-T therapy are CD19, CD20, CD38, and BCMA, which are expressed on different B cell subsets. These cells are responsible for producing autoantibodies that drive diseases such as SLE, RA, and MS. CAR-T cells can eliminate these B cells with greater depth and durability than monoclonal antibodies, and they persist in the body, reducing the likelihood of relapse.
Targeting Autoreactive T Cells
Autoreactive T cells contribute to diseases like Crohn’s disease and ulcerative colitis by directly damaging tissues or helping B cells produce autoantibodies. CAR-T cells targeting CD7 and CD70, both present on T cells, are now in early clinical testing (e.g., NCT05239702). These approaches may help eliminate pathogenic T cells while sparing normal immunity.
The Role of CAR-Tregs
Another breakthrough is the engineering of CAR-T regulatory cells (CAR-Tregs). These cells don’t destroy other immune cells—instead, they suppress immune overactivation and restore immune tolerance. CAR-Tregs are being developed for MS, T1DM, and transplantation, and they may offer long-term control with fewer side effects.
In summary, CAR-T therapies can now either eliminate disease-driving cells or modulate immune function, opening multiple therapeutic pathways for precision treatment in autoimmunity.
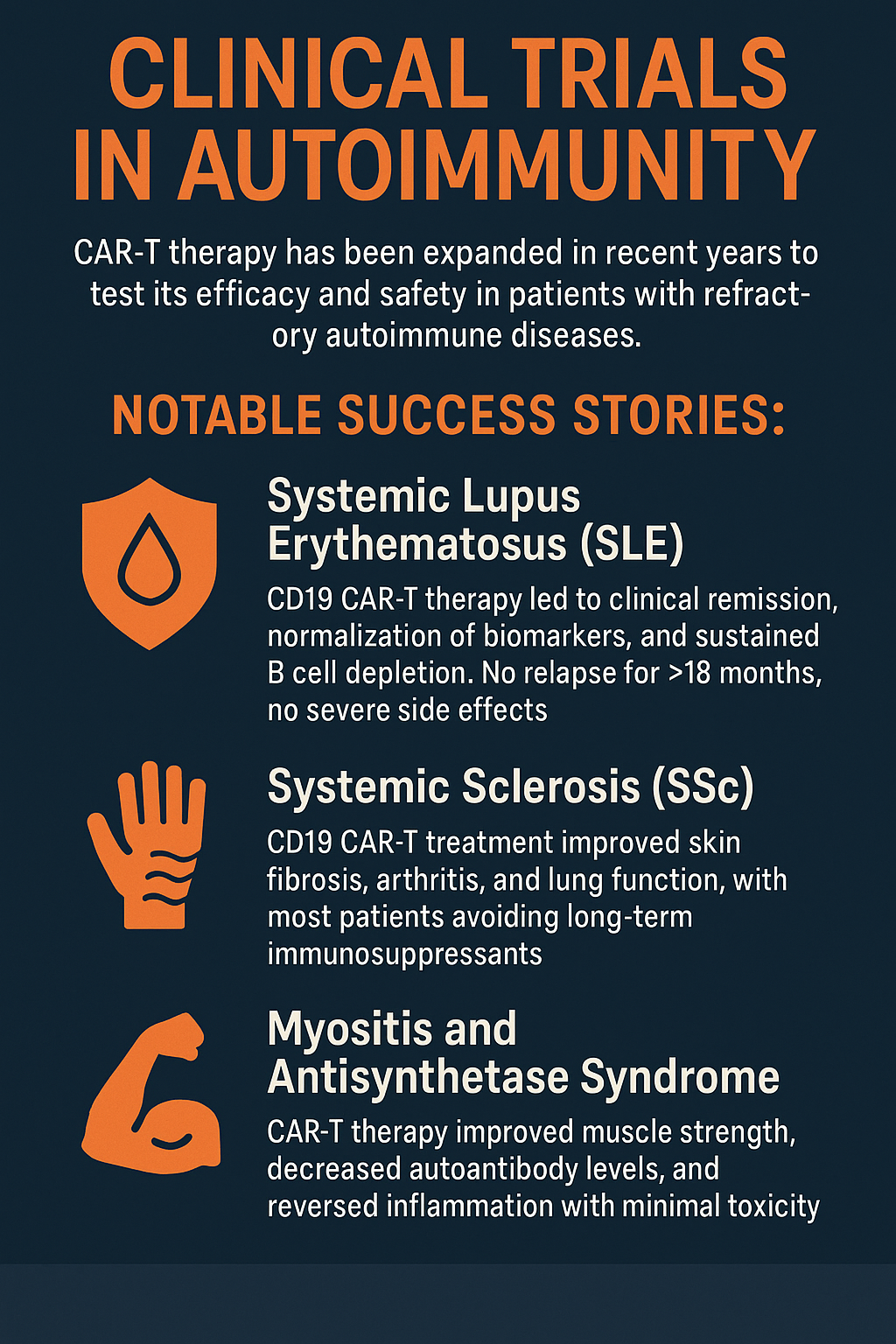
Clinical Trial Highlights – From Lupus to Myositis
CAR-T therapy is no longer confined to the lab. Clinical trials in recent years have expanded to test its efficacy and safety in patients with refractory autoimmune diseases, where traditional treatments have failed.
Notable Success Stories:
In systemic lupus erythematosus (SLE), CD19 CAR-T therapy led to clinical remission, normalization of biomarkers, and sustained B cell depletion. One study reported no relapse for over 18 months with no severe side effects.
For systemic sclerosis (SSc), CD19 CAR-T treatment improved skin fibrosis, arthritis, and lung function, with most patients avoiding long-term immunosuppressants.
In myositis and antisynthetase syndrome, CAR-T therapy improved muscle strength, decreased autoantibody levels, and reversed inflammation with minimal toxicity.
Dosing & Protocols:
Most autoimmune trials use CAR-T cell doses ranging from 1–3 × 10⁶ cells/kg, with lymphodepletion via fludarabine and cyclophosphamide to enhance CAR-T engraftment. This approach mirrors protocols from oncology, ensuring predictable expansion and persistence.
Safety Profile:
Unlike in cancer, where cytokine release syndrome (CRS) and neurotoxicity are common, autoimmune patients showed minimal adverse events—typically grade 1 CRS or none at all. This improved safety is likely due to the lower immune burden compared to tumors.
These trials mark a pivotal shift: CAR-T is no longer just experimental—it’s becoming a viable option for difficult-to-treat autoimmune cases.
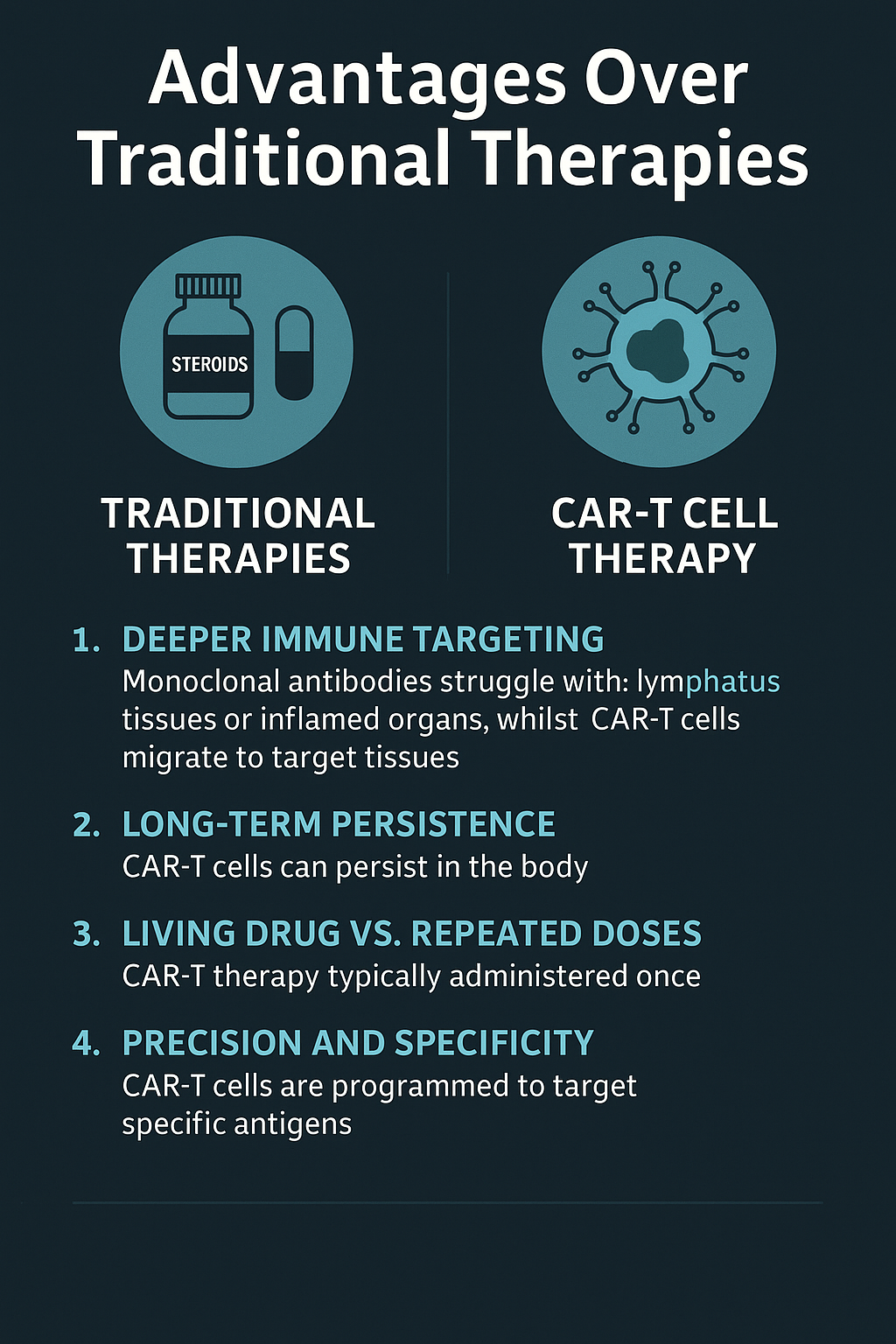
Advantages Over Traditional Therapies
Traditional treatments for autoimmune diseases—such as corticosteroids, monoclonal antibodies (mAbs), and disease-modifying antirheumatic drugs (DMARDs)—have helped manage symptoms, but they often come with significant limitations. These include systemic immunosuppression, repeated dosing, and limited access to tissue-resident immune cells.
By contrast, CAR-T cell therapy offers a transformative approach. Here’s how it stands apart:
1. Deeper Immune Targeting
Monoclonal antibodies (like rituximab or ocrelizumab) can deplete B cells in the bloodstream but struggle to penetrate lymphatic tissues or inflamed organs. CAR-T cells, being living entities, can migrate to target tissues, leading to more comprehensive immune modulation.
2. Long-Term Persistence
CAR-T cells can persist in the body for months or even years, maintaining surveillance against autoreactive cells. This reduces the need for chronic therapy and increases the likelihood of sustained remission.
3. Living Drug vs. Repeated Doses
Unlike biologics requiring monthly infusions, CAR-T therapy is typically administered once, with effects lasting long-term due to the T cells’ capacity to expand and form memory subsets.
4. Precision and Specificity
CAR-T cells are genetically programmed to target specific surface antigens, minimizing off-target effects and systemic toxicity compared to broader immunosuppressive regimens.
While CAR-T therapy comes with its own risks, including cytokine release syndrome (CRS), these events have been relatively rare and mild in autoimmune trials. As such, the risk–benefit ratio increasingly favors CAR-T, especially in refractory disease.
Challenges and future prospects
Despite the bright prospects, CAR-T therapy still needs to overcome three major difficulties:
1. Safety optimization
– “Suicide switch” design: adding apoptosis-inducing genes through gene editing, CAR-T cells can be quickly eliminated when necessary.
– Local administration: developing in situ CAR-T therapy for specific organs (such as joints and intestines) to reduce systemic toxicity.
2. Cost and accessibility
– “Off-the-shelf” CAR-T: using gene editing technology to knock out T cell HLA molecules and develop universal products, the cost can be reduced by 80%.
– In vivo programming technology: directly delivering CAR genes to T cells in vivo through lipid nanoparticles (LNP) to avoid in vitro culture (Capstan’s CPTX2309 has entered clinical trials).
3. Durability of efficacy
– Combination therapy: CAR-T is combined with IL-2 sustained-release agents and PD-1 inhibitors to enhance T cell persistence. – Directed expansion of memory T cells: by optimizing the costimulatory domain (such as 4-1BB), CAR-T cells are promoted to differentiate into memory phenotypes.
CAR-T therapy is reshaping the treatment paradigm of autoimmune diseases:
– Disease modification: from “controlling symptoms” to “curing the cause”, some patients may achieve lifelong remission.
– Personalized medicine: Customizing CAR according to patient-specific antigens (such as idiotypic autoantibodies) to achieve “one person, one policy”.
– Cross-border application: CAR-Treg combined with stem cell transplantation is expected to solve the rejection reaction after organ transplantation.
Conclusion: A New Era for Autoimmune Disease Treatment
CAR-T cell therapy is rapidly emerging as a game-changer in the treatment of autoimmune diseases. Originally designed to combat cancer, this powerful technology is now being adapted to precisely target autoreactive B and T cells, the root drivers of many autoimmune conditions. By offering deep immune cell depletion or immunosuppressive regulation through CAR-Tregs, this approach holds the potential to induce lasting remission with minimal systemic toxicity.
Unlike traditional treatments such as monoclonal antibodies or immunosuppressants, CAR-T cells act as a “living drug”, capable of expanding, persisting, and maintaining immune surveillance. Early clinical trials in diseases like systemic lupus erythematosus, myositis, and systemic sclerosis have shown remarkable results, including sustained symptom relief and few side effects.
However, challenges remain—such as high costs, manufacturing delays, and the need for further safety validation. Still, innovations like off-the-shelf allogeneic CAR-T products and refined dosing strategies are on the horizon.
In short, CAR-T therapy is not just a treatment—it represents a transformative step toward curing autoimmune diseases. As science progresses, this once-oncology-exclusive tool is poised to become a mainstay in immunotherapy for chronic inflammatory conditions.
Reference
Li YR, Lyu Z, Chen Y, Fang Y, Yang L. Frontiers in CAR-T cell therapy for autoimmune diseases. Trends Pharmacol Sci. 2024 Sep;45(9):839-857. doi: 10.1016/j.tips.2024.07.005. Epub 2024 Aug 14. PMID: 39147651.
https://www.cell.com/trends/pharmacological-sciences/article/S0165-6147(24)00147-0/pdf




