Expanding Horizons: Vericiguat's Potential in Cardiovascular Medicine and Future Directions
Abstract
Vericiguat, a novel soluble guanylate cyclase (sGC) stimulator, has emerged as a promising therapeutic agent in cardiovascular medicine. With its unique mechanism of action, vericiguat holds potential for addressing various cardiovascular conditions. Understanding its mechanism of action is crucial for elucidating its therapeutic effects and potential applications. By targeting sGC and enhancing cyclic guanosine monophosphate (cGMP) signaling, vericiguat modulates key pathways involved in vascular tone regulation, myocardial function, and remodeling. The clinical and translational significance of vericiguat extends beyond its immediate therapeutic implications, offering opportunities for exploring new therapeutic avenues and refining existing treatment strategies.
Heart failure (HF) presents a growing global health challenge, affecting over 64 million adults and causing 9.9 million deaths annually. Despite conventional treatments like mineralocorticoid receptor antagonists and ACE inhibitors, the prognosis remains poor, necessitating novel therapeutic approaches. Vericiguat, an oral soluble guanylate cyclase (sGC) stimulator, gained FDA approval in 2021 as the first chronic HF treatment. By enhancing cGMP activity, vericiguat modulates cardiovascular actions, reducing HF hospitalizations and cardiovascular deaths.
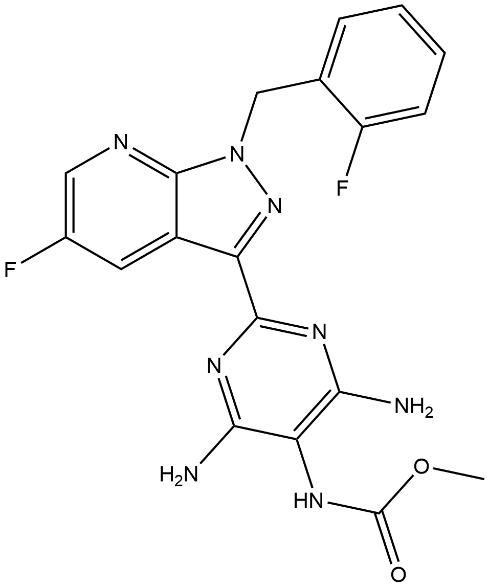
Fig 2. The Structural formula of Vericiguat
Vericiguat represents a novel therapeutic agent with promising implications in various cardiovascular conditions. Its mechanism of action lies in the modulation of the cyclic guanosine monophosphate (cGMP) pathway, a key player in cardiovascular regulation. At the heart of its mechanism is the sGC enzyme, which serves as the primary target for vericiguat. sGC is a heterodimeric enzyme complex predominantly found in smooth muscle cells. Upon activation by various stimuli, including nitric oxide (NO), sGC catalyzes the conversion of guanosine triphosphate (GTP) to cGMP. This cyclic nucleotide acts as a secondary messenger, orchestrating diverse physiological responses within the cardiovascular system.
The action mechanism of vericiguat
Vericiguat exerts its effects by enhancing the activity of sGC, thereby amplifying cGMP production. This activation occurs independently of NO, offering a distinct advantage over conventional NO-dependent sGC stimulators. By potentiating sGC function, vericiguat augments intracellular cGMP levels, leading to downstream signaling cascades.
The signaling pathways modulated by vericiguat encompass both cGMP-dependent and NO-mediated pathways. Firstly, increased cGMP levels activate protein kinase G (PKG), which regulates various cellular processes such as smooth muscle relaxation, ion channel conductance, and gene expression. Secondly, vericiguat enhances NO sensitivity by stabilizing the interaction between sGC and NO, thereby amplifying NO-mediated cGMP production.
Beyond these classical pathways, vericiguat exerts additional downstream effects that contribute to its therapeutic efficacy. These effects include the inhibition of platelet aggregation, reduction of fibrosis, and modulation of cardiac remodeling processes. Such pleiotropic actions underscore the multifaceted nature of vericiguat’s pharmacology and its potential to address complex cardiovascular pathologies.
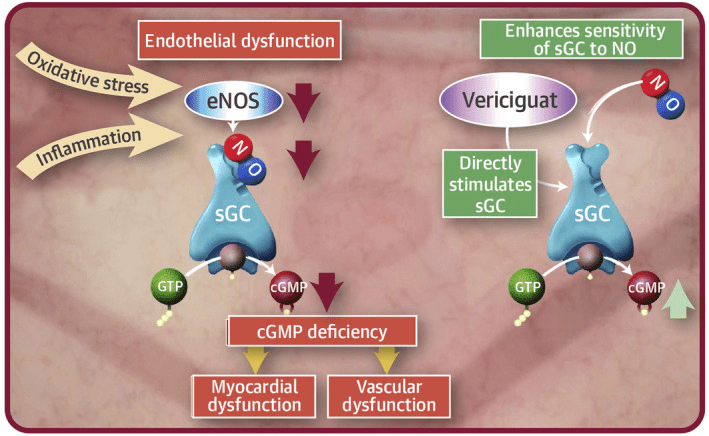
Fig 2. Mechanism of action of vericiguat [1].
The clinical trials involving vericiguat
Vericiguat has undergone extensive evaluation in various clinical trials to assess its efficacy and safety in different patient populations. These trials have aimed to elucidate its potential therapeutic benefits across a spectrum of cardiovascular conditions. Efficacy outcomes observed in clinical trials involving vericiguat have demonstrated promising results. Studies have shown significant improvements in key clinical endpoints such as cardiovascular mortality, hospitalization for heart failure, and exercise capacity. Additionally, vericiguat has exhibited favorable effects on surrogate markers of cardiovascular health, including reductions in natriuretic peptides and improvements in echocardiographic parameters.
The safety and tolerability profile of vericiguat observed in clinical settings have been generally favorable. Common adverse events reported include headache, hypotension, and gastrointestinal symptoms, although these are typically mild to moderate in severity. Importantly, serious adverse events, such as worsening renal function and hypotension requiring intervention, have been infrequent and manageable with appropriate monitoring and dose adjustments. Overall, vericiguat has demonstrated a favorable benefit-risk profile in clinical trials, supporting its potential as a valuable therapeutic option for patients with cardiovascular diseases.
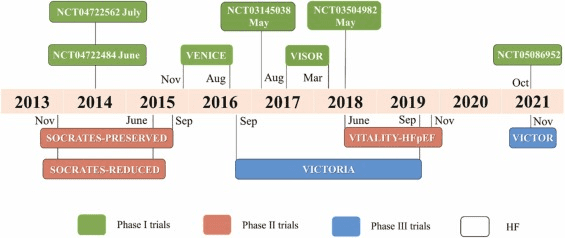
Fig 3. The clinical trial timeline of vericiguat [4].
The future opportunities and challenges
Potential applications beyond initial indications for vericiguat are vast and intriguing. While initially developed and approved for the treatment of heart failure, its pharmacological properties suggest broader therapeutic potential. Vericiguat’s mechanism of action, targeting soluble guanylate cyclase (sGC) to enhance cyclic guanosine monophosphate (cGMP) signaling, opens avenues for exploring its efficacy in other cardiovascular conditions such as pulmonary hypertension, coronary artery disease, and peripheral vascular disease. Moreover, considering its ability to modulate vascular tone and improve cardiac function, vericiguat may hold promise in conditions characterized by endothelial dysfunction and impaired vasodilation. Additionally, its favorable safety profile suggests potential applications in elderly populations or those with multiple comorbidities who may be at higher risk of adverse effects with traditional therapies.
Challenges and opportunities for further research with vericiguat abound. While initial clinical trials have demonstrated promising results, further investigation is needed to elucidate its long-term efficacy and safety, particularly in diverse patient populations. Addressing gaps in knowledge, such as the impact of vericiguat on specific patient subgroups, optimal dosing strategies, and potential drug interactions, requires rigorous clinical studies and real-world evidence. Moreover, exploring novel biomarkers predictive of treatment response and refining patient selection criteria could enhance therapeutic outcomes and aid in personalized medicine approaches.
Future directions in clinical and translational studies involving vericiguat encompass several key areas. Firstly, investigating its potential synergistic effects with existing therapies, such as SGLT2 inhibitors and ARNIs, could optimize treatment strategies for heart failure patients. Secondly, exploring the role of vericiguat in combination therapies for other cardiovascular conditions holds promise for improving patient outcomes. Additionally, leveraging advanced imaging techniques and biomarker assessments could provide valuable insights into vericiguat’s mechanism of action and treatment response. Lastly, conducting large-scale, multicenter trials with diverse patient populations will be essential for establishing vericiguat’s efficacy and safety across a spectrum of cardiovascular diseases, ultimately shaping its future clinical utility.
Reference
- Trujillo, M. E., Ayalasomayajula, S., Blaustein, R. O., & Gheyas, F. (2023). Vericiguat, a novel sGC stimulator: Mechanism of action, clinical, and translational science. Clinical and translational science, 16(12), 2458–2466.
- Shaikh, T. G., Jawed, S., Rahmat, Z. S., Ahmed, S. H., Waseem, S., Ullah, I., Irfan, M., & Asghar, M. S. (2023). Efficacy and Safety of Vericiguat for Treatment of Heart Failure: A Systematic Review. Current problems in cardiology, 48(5), 101586.
- Vericiguat for chronic heart failure. (2023). Australian prescriber, 46(4), 98–99.
- Xia, J., Hui, N., Tian, L., Liang, C., Zhang, J., Liu, J., Wang, J., Ren, X., Xie, X., & Wang, K. (2022). Development of vericiguat: The first soluble guanylate cyclase (sGC) stimulator launched for heart failure with reduced ejection fraction (HFrEF). Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 149, 112894.