Enlicitide in Hyperlipidemia: Mechanism, Clinical Evidence, and Future Outlook
Abstract
Hyperlipidemia, characterized by elevated LDL-C levels, is a major global health concern and a key contributor to atherosclerotic cardiovascular disease (ASCVD). While lifestyle modifications are foundational in managing lipid levels, pharmacologic intervention is often necessary for high-risk individuals. Enlicitide, a novel oral PCSK9 inhibitor, offers a promising therapeutic option by inhibiting PCSK9 and enhancing LDL receptor activity, thereby lowering LDL-C effectively. Phase III clinical trials, including CORALreef HeFH and CORALreef AddOn, have demonstrated enlicitide’s superior efficacy and favorable safety profile compared to placebo and other lipid-lowering agents. The development of oral PCSK9 inhibitors like enlicitide marks a significant advancement over traditional injectable biologics, offering enhanced patient compliance and convenience. If approved, enlicitide would become the first oral PCSK9 inhibitor in the U.S., reshaping the hyperlipidemia treatment landscape. Merck’s ongoing CORALreef program will further evaluate its long-term impact on lipid management and cardiovascular outcomes across diverse populations.
About Hyperlipidemia
Hyperlipidemia is a metabolic disorder defined by elevated levels of low-density lipoprotein cholesterol (LDL-C) in the bloodstream, typically resulting from an excess of lipids or fats. In the United States, this condition presents a substantial public health concern, affecting approximately 120 million adults—nearly 40% of the adult population. Alarmingly, an estimated 28.5 million of these individuals have LDL-C levels exceeding 240 mg/dL, placing them in a high-risk category for serious health complications.
One of the most significant consequences of hyperlipidemia is its role as a primary risk factor for atherosclerotic cardiovascular disease (ASCVD). ASCVD includes conditions such as coronary artery disease and stroke, which collectively account for 78% of all cardiovascular-related deaths worldwide. The pathogenesis of ASCVD is strongly linked to elevated LDL-C, which directly contributes to the formation of atherosclerotic plaques within arterial walls. In addition, hypertriglyceridemia—present in roughly 31% of American adults—exacerbates this risk by promoting vascular injury through the accumulation of triglyceride-rich lipoprotein remnants.
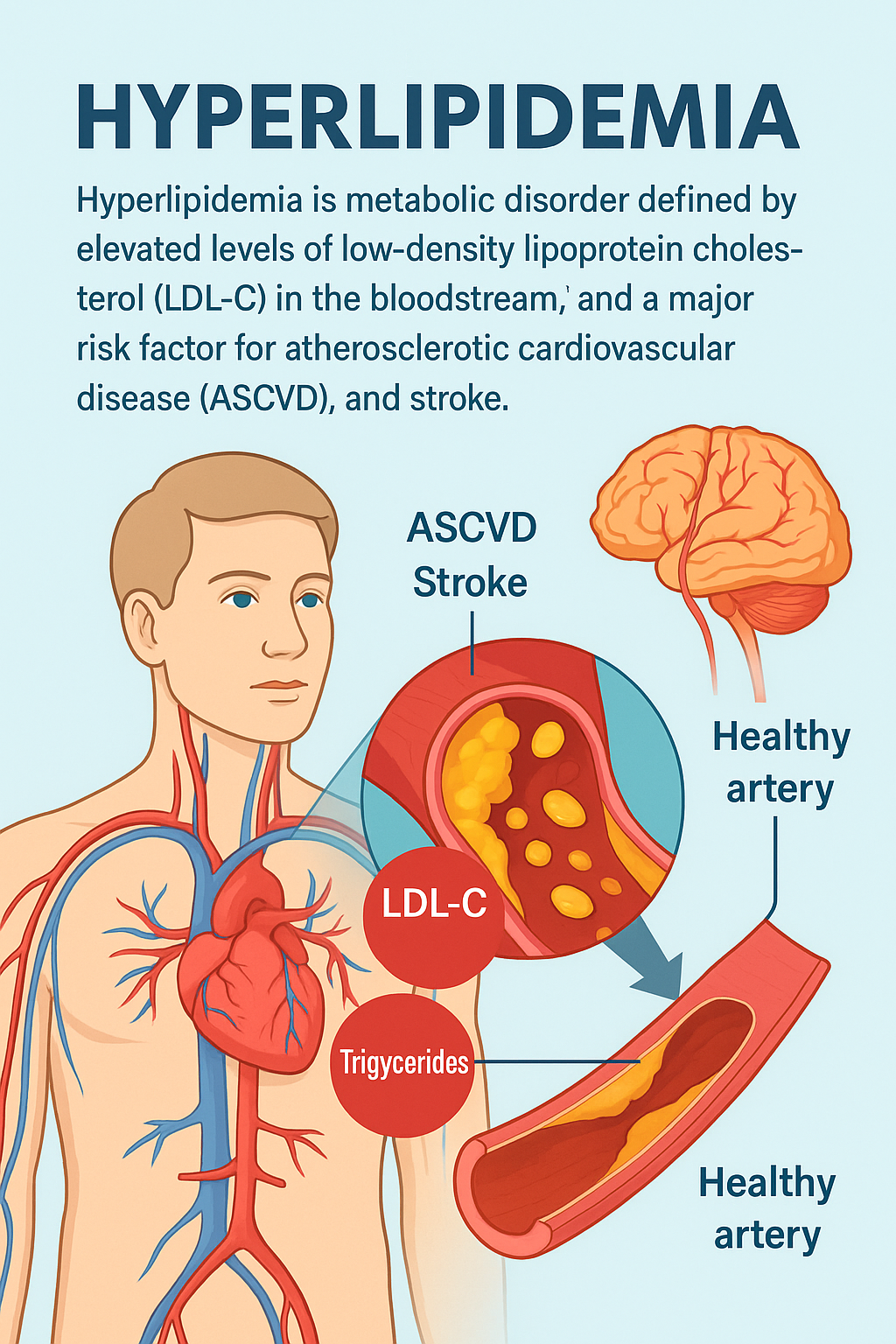
While lifestyle modifications such as a heart-healthy diet, increased physical activity, and weight management are foundational in controlling lipid levels, they may not be sufficient for everyone. A considerable portion of the population remains unable to reach target cholesterol thresholds through lifestyle changes alone. For these individuals, pharmacological intervention becomes essential. Drug therapies, including statins, PCSK9 inhibitors, and other lipid-lowering agents, play a critical role in reducing LDL-C levels and mitigating the risk of cardiovascular events.
Addressing hyperlipidemia effectively requires a multifaceted approach that combines preventive strategies with evidence-based medical treatments to reduce the global burden of cardiovascular disease.
Mechanism of Action
Enlicitide is a next-generation oral PCSK9 inhibitor designed to lower low-density lipoprotein cholesterol (LDL-C) by targeting a critical regulatory pathway in lipid metabolism. It works by specifically binding to proprotein convertase subtilisin/kexin type 9 (PCSK9), thereby preventing its interaction with the low-density lipoprotein receptor (LDL-R).
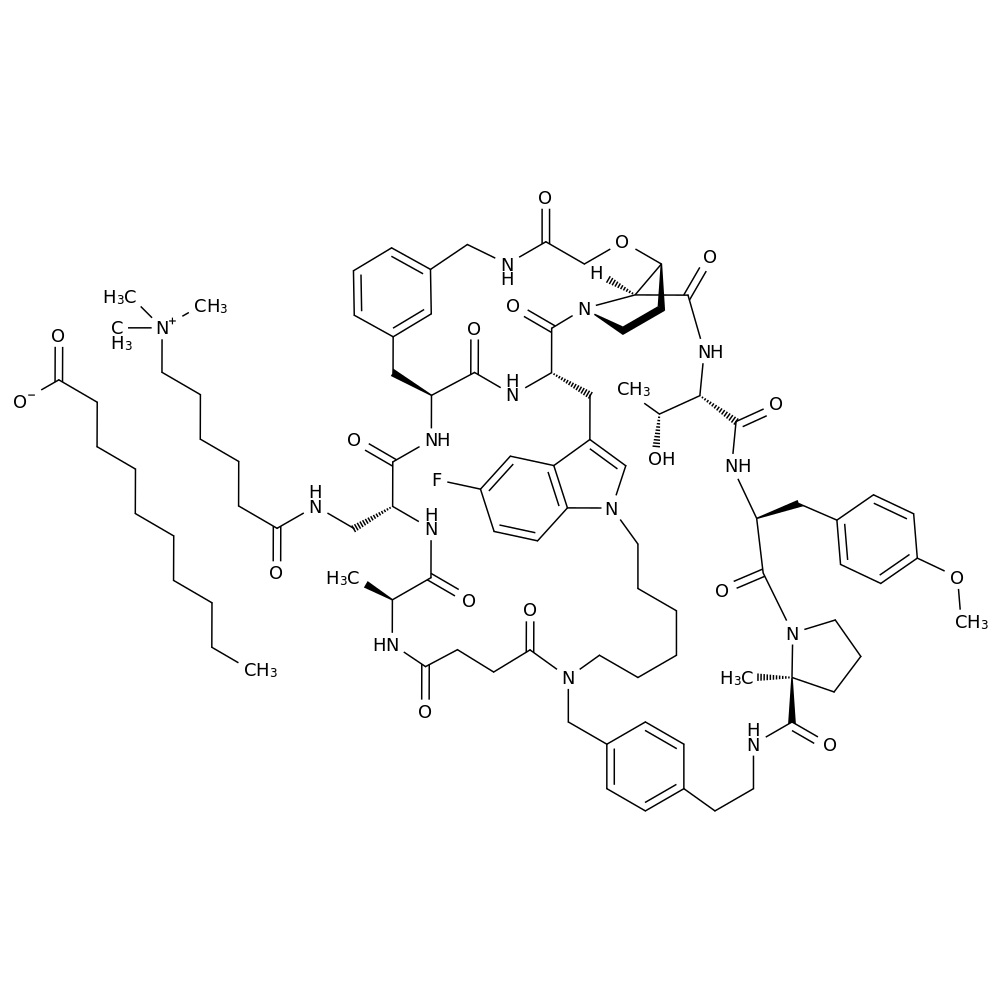
Enlicitide structural formula
PCSK9 plays a central role in cholesterol homeostasis by regulating the degradation of LDL receptors on hepatocyte surfaces. Under normal conditions, PCSK9 binds to LDL-R, leading to their internalization and lysosomal degradation, which limits the liver’s ability to remove LDL-C from the bloodstream. Enlicitide interrupts this process, allowing more LDL receptors to remain active on the cell surface.
By preserving and increasing LDL-R availability, Enlicitide enhances the clearance of circulating LDL-C, resulting in significantly reduced blood cholesterol levels. This mechanism provides a promising oral therapeutic alternative to existing injectable PCSK9 inhibitors, offering both efficacy and convenience in managing hyperlipidemia and reducing cardiovascular risk.
Clinical Trial Data
Enlicitide has demonstrated robust efficacy and safety in multiple Phase III clinical trials targeting patients with hypercholesterolemia and elevated cardiovascular risk.
The CORALreef HeFH (NCT05952869) trial is a Phase III, randomized, double-blind, placebo-controlled, multicenter study evaluating enlicitide in adults with heterozygous familial hypercholesterolemia (HeFH). Participants, all on moderate- or high-intensity statins with or without additional lipid-lowering therapies, had established or high risk for atherosclerotic cardiovascular disease (ASCVD). The primary endpoints included the mean percentage change in LDL-C at week 24, incidence of adverse events (AEs), and discontinuations due to AEs. Enlicitide significantly reduced LDL-C levels versus placebo and showed meaningful improvements in secondary endpoints, including non-HDL-C, ApoB, and Lp(a) at week 24 and LDL-C at week 52.
The CORALreef AddOn (NCT06450366) study compared enlicitide with ezetimibe, bempedoic acid, and their combination. Enlicitide demonstrated superior LDL-C reduction at week 8 and a comparable safety profile. Adverse events and serious adverse events (SAEs) were not significantly different from those in the comparator groups.
Progress in the Development of PCSK9 Inhibitors
Significant strides have been made in the development of PCSK9 inhibitors as an effective class of lipid-lowering therapies. To date, seven PCSK9 inhibitors have received regulatory approval, most notably evolocumab and alirocumab. These agents are primarily monoclonal antibodies designed to bind circulating PCSK9, thereby preventing its interaction with LDL receptors. Administered via subcutaneous injection, they have demonstrated profound efficacy in reducing cardiovascular risk.
Among them, evolocumab has shown exceptional results across multiple Phase III clinical trials, consistently lowering LDL-C by more than 50% compared to placebo. Beyond LDL-C reduction, these biologics also positively influence a range of secondary lipid parameters. Benefits include reductions in non-HDL-C, apolipoprotein B (ApoB), triglycerides (TG), and lipoprotein(a) [Lp(a)], along with modest increases in high-density lipoprotein cholesterol (HDL-C) and apolipoprotein A1 (ApoA1).
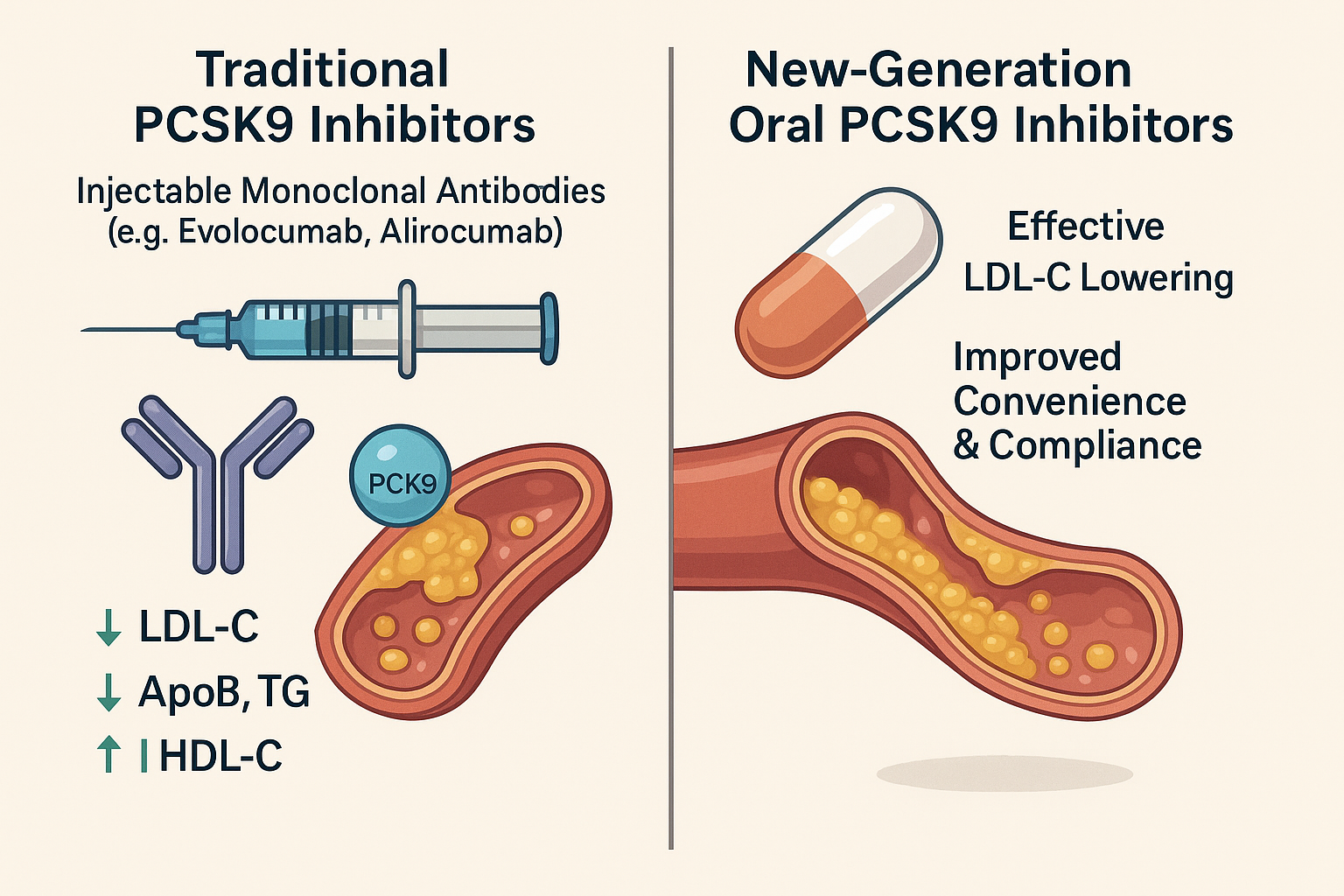
Building on this foundation, new-generation therapies such as enlicitide and other oral PCSK9 inhibitors are currently under development. These novel agents aim to improve patient convenience and compliance by offering effective LDL-C lowering without the need for injectable administration. The shift toward oral formulations represents a transformative advancement in lipid management, potentially expanding accessibility and adherence for broader patient populations.
Outlook
The positive outcomes of the Phase III clinical trials for Enlicitide mark a pivotal advancement in the treatment of hyperlipidemia, particularly for patients who are either intolerant to existing therapies or do not achieve adequate lipid control. As an oral PCSK9 inhibitor, Enlicitide has the potential to significantly enhance treatment adherence by eliminating the need for injectable administration, thereby improving LDL-C reduction and long-term cardiovascular outcomes.
If approved, Enlicitide would become the first oral PCSK9 inhibitor available in the United States, representing a major milestone in lipid management and offering Merck a strategic edge in the competitive cardiovascular therapeutics market. Its introduction is poised to reshape the hyperlipidemia treatment landscape, encouraging the adoption of oral therapies and broadening options for both clinicians and patients.
Merck continues to invest in its CORALreef clinical program, which includes two additional ongoing studies: CORALreef Lipids and CORALreef Outcomes. These trials aim to further assess the long-term safety, efficacy, and cardiovascular event prevention potential of Enlicitide. Future research will also explore synergistic effects when used in combination with other lipid-lowering therapies and its utility across diverse patient populations, setting the stage for broader clinical integration and global impact in the management of dyslipidemia.
Conclusion
Enlicitide represents a significant advancement in lipid management, offering an effective and convenient oral alternative to injectable PCSK9 inhibitors. By enhancing LDL receptor availability and substantially lowering LDL-C, it addresses the unmet needs of patients with hyperlipidemia who struggle with current treatment options. Its strong clinical performance in Phase III trials, coupled with a favorable safety profile, supports its potential to reshape the therapeutic landscape. If approved, Enlicitide would become the first oral PCSK9 inhibitor in the U.S., marking a new era in cardiovascular disease prevention and expanding patient access to innovative lipid-lowering therapy.
References
Li H, Thaisrivongs DA, Shang G, Chen Y, Chen Q, Tan L, Xiao KJ, Larson RT, Kuethe JT, Lee J, Deprez NR, Nolting AF, Poirier M, Bulger PG, Regalado EL, Biba M, Tsay FR, DaSilva J, Prier CK, Strulson CA, Zawatzky K, Liu Z, Newman JA, Sokolowsky K, Tang W, Hullen K, Thakur N, Welch C, Patel S, He Y, Xu J, Variankaval N, Klapars A, Kong J, Desmond R, Varsolona R, Maligres PE, Pons Siepermann CA, Robison L, Piou T, Hartmanshenn C, Chandra A, Patel A, Becker MR, Liu G, Duan J, Wan B, Xiao C, Yuan Y, Cao X, Chen L, Yi R, Wu Z, Feng M, Li D, Song Z, Dong Y, Sun J, Li B, Shao G, Campeau LC, Yin J. Total Synthesis of Enlicitide Decanoate. J Am Chem Soc. 2025 Apr 2;147(13):11036-11048. doi: 10.1021/jacs.4c15966. Epub 2025 Mar 24. PMID: 40123407.
https://pubs.acs.org/doi/abs/10.1021/jacs.4c15966
Campeau LC. From Cortisone to Enlicitide: A Journey of Synthetic Chemistry Innovations at Merck. J Org Chem. 2025 Apr 11;90(14):4781-4795. doi: 10.1021/acs.joc.4c02919. Epub 2025 Apr 1. PMID: 40168664; PMCID: PMC11998012.
https://pubs.acs.org/doi/full/10.1021/acs.joc.4c02919
Tokgözoğlu L, Pirillo A, Catapano AL. Oral PCSK9 Inhibitors: Will They Work? Curr Atheroscler Rep. 2025 Apr 30;27(1):53. doi: 10.1007/s11883-025-01299-7. PMID: 40304930.
https://link.springer.com/article/10.1007/s11883-025-01299-7
Ferri N, Marodin G. Emerging oral therapeutic strategies for inhibiting PCSK9. Atheroscler Plus. 2024 Dec 12;59:25-31. doi: 10.1016/j.athplu.2024.11.003. PMID: 39802651; PMCID: PMC11722601.
https://www.sciencedirect.com/science/article/pii/S266708952400052X