Drug Impurity and Check
Abstract
Drug impurities have a direct impact on drug quality, safety, and stability. Controlling these impurities is crucial for maintaining drug efficacy and minimizing adverse reactions. This paper reviews impurity sources, their effects on drug safety, and methods for control and inspection. It emphasizes the importance of studying drug impurities and their management.
Introduction
Drug impurities are substances that lack therapeutic effects or can compromise drug stability, efficacy, and even human health. Throughout the research, production, storage, and clinical use of drugs, it is imperative to uphold drug purity and minimize impurities to ensure drug effectiveness and safety. Drug purity assessment encompasses the evaluation of structural integrity, physical characteristics, chemical constants, impurity levels, and content determination as interconnected components. The presence of impurities in a drug is the primary factor affecting its purity. Exceeding impurity limits can alter physical and chemical properties, change appearance, and impact drug stability. Elevated impurity levels invariably reduce drug content, diminish activity, and significantly increase toxic side effects. Therefore, impurity testing is a crucial step in controlling drug purity and enhancing drug quality. Advancements in separation and detection technologies have enabled a deeper exploration of drug purity, allowing us to uncover the impact of certain impurities on drug efficacy and potential side effects. As raw materials quality and production methods continue to improve, the standards for impurity inspection in drugs have also been raised to meet these higher quality expectations.
Where does the impurity come from?
1.1 Introduction to Production Impurities
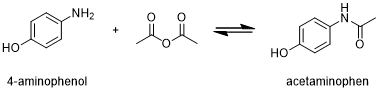
Figure 1 The preparation of acetaminophen
- Raw Material-Related Impurities: Impurities in drug raw materials can arise from various sources, including incomplete reactions during synthesis, the presence of intermediates or by-products, and the purity of starting materials. For instance, in the production of acetaminophen, if the acetylation reaction is reversible, residual 4-aminophenol may be present in the final product[1]. Similarly, trace amounts of cholic acid in ursodeoxycholic acid can be attributed to the raw material’s insufficient purity.
- Solvent-Related Impurities: Impurities in drugs can also result from solvent residues, including solutes and catalysts, used during raw material production. For example, during the purification of ampicillin, different organic solvents may lead to varying degrees of residue, primarily dichloromethane, isopropyl alcohol, and acetone. In the production of Amikacin, multiple ethanol recrystallization and refinement steps may result in residual ethanol in the raw material.[2]
1.2 Introduction During Storage
- Environmental Factors and Microorganisms: Impurities can be generated during drug storage due to external conditions such as temperature, humidity, sunlight, and exposure to air or microbial action. For instance, anesthetic ether can oxidize into toxic peroxides when exposed to air during storage. Prolonged or improper storage of calomel can lead to the formation of toxic mercury compounds. Mebendazole has three crystal forms (A, B, and C), with C being the effective form, while A is ineffective. Therefore, any crystal forms other than C may be considered impurities.
- Degradation Reactions: Drug degradation can occur during storage, resulting in the formation of degradation products through processes like hydration, oxidation, and ring cracking. For instance, enalapril’s ester group can hydrolyze into enalapril, which can further form Diketopiperazines.
- Excipients in Pharmaceutical Preparations: Some drug impurities may arise from the excipients specified in pharmaceutical formulations. For example, certain drugs contain impurities like methylparaben, and the capsule shell’s preservative components can also generate impurities. These impurities are identified through various analytical methods such as LC-MS, GC-MS, and UV, and are produced due to interactions between the main drug and small amounts of succinic acid and benzoic acid present in the excipients.
1.3 Other Factors Contributing to Drug Impurities
Various factors beyond those mentioned earlier can introduce drug impurities, including:
- Decay in radiopharmaceuticals.
- Abnormal expression of proteins in biological products.
- The presence of optical isomers in chiral compounds.
- Pesticide residues in Chinese herbal medicine preparations.
The Impact of Impurities On Drug Safety
The connection between drug impurities and drug safety is intricate and influenced by numerous factors. Most impurities in drugs typically possess potential biological activity, with some even interacting with the drug to impact its efficacy and safety, potentially leading to severe toxic effects.
2.1 Toxic Side Effects of Drug Impurities
Certain drug impurities can lead to toxic side effects. For example, the immunogenic penicillithiazole protein, generated by the action of the β-lactam ring, acts as an exogenous allergen. Additionally, the self-polymerization of a lactam ring during storage produces macromolecular polymers, which act as endogenous allergens. These factors contribute to the propensity of β-lactam antibiotics to trigger allergic reactions.
Figure 2 The structure of MPTP
In the case of heterocyclic drugs, one of the most common synthetic impurities is N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine(MPTP)[3], which selectively damages dopaminergic neurons in the nigra and pallidum, leading to symptoms resembling Parkinson’s disease. Tetracyclines can produce degradation products that cause Fanconi syndrome, and methotrexate by-products can induce febrile reactions.
2.2 Impact of Chiral Compound Optical Isomers on Drug Safety
Chiral compounds exhibit varying effects on drug safety. Some chiral compounds have enantiomers with identical pharmacological effects but differing potencies, while others have complementary effects. However, in most cases, the optical isomers of drugs can significantly influence drug efficacy, and in some instances, lead to severe adverse reactions.
Researchers have noted the following ways in which chiral compound optical isomers can affect drug efficacy:
- Reduced Drug Efficacy: For instance, the racemic mixture of the quinolone antibiotic ofloxacin is only half as effective as its left enantiomer.
- Opposing Pharmacological Effects: For example, the R-enantiomer of Zachopili acts as a 5-HT3 receptor antagonist, while the S-enantiomer serves as a 5-HT3 receptor agonist.
- Induction of Serious Adverse Reactions: Thalidomide’s R-isomer and its two metabolites exhibit strong embryotoxicity and teratogenic effects.
These variations underscore the importance of considering chiral compound optical isomers in drug safety assessments.
Impurity Control
All drug impurities have varying degrees of impact on drug stability and safety. Therefore, it is crucial to rigorously control the levels of drug impurities during both drug production and storage. Impurity inspection serves as a critical quality control measure for drugs. This inspection can be categorized into two types: general impurity inspection and special impurity inspection.
3.1 General Impurity Inspection
General impurity inspection methods and limits are specified by pharmacopeias. These methods encompass the examination of various elements and characteristics, including chlorides, sulfates, sulfides, selenium, fluorine, cyanide, iron salts, heavy metals, arsenic salts, ammonium salts, pH levels, clarity, solution color, drying weight loss, moisture content, incandescence residue, carbonized substances, organic solvent residues, and others.[4]
3.2 Special Impurity Inspection
Special impurities typically result from factors such as the nature of drugs, production methods, and technological conditions during drug production and storage. Given the diverse nature of these impurities, inspection methods may vary. Commonly employed methods include:
- Physical Method: This involves assessing differences in properties like smell, taste, volatility, color, solubility, and optical activity between drugs and impurities. The goal is to ensure impurity levels are within acceptable limits.
- Chemical Reaction Method: This encompasses volumetric analysis, gravimetric analysis, colorimetry, and turbidimetry.
- Chemical Analysis: Methods such as ultraviolet spectrophotometry, capillary zone electrophoresis (CzE), and high-performance capillary electrophoresis (HPCE) are commonly used. For instance, ultraviolet spectrophotometry measures cytidine disodium triphosphate absorption at 280nm/260nm, with an acceptable ratio of 2.00 to 2.20.[5] HPCE is used to determine z and E isomers in Toremifene citrate and impurities in drugs like tetracycline, tetracycline, and aureomycin.
- Chromatography: Chromatography is the most widely used and effective method for drug impurity analysis. It offers high sensitivity, accuracy, simplicity, speed, and efficiency. It’s increasingly incorporated into pharmacopeias for impurity control due to its numerous advantages. Various chromatography techniques are employed for drug impurity analysis. These techniques differ in their suitability and application scope:
- Paper Chromatography (P.C): P.C involves applying a limited impurity control solution onto chromatographic filter paper and comparing the number, color, or fluorescence intensity of impurity spots after development. This method is typically used for drugs or radiopharmaceuticals with high polarity. However, it has limitations, including long development times, diffuse spots, and difficulty in developing corrosive colors with strong acids, restricting its use.
- Thin-Layer Chromatography (TLC): TLC, similar to P.C, is widely used in drug impurity inspections due to its simplicity, minimal equipment requirements, and extensive use in pharmacopoeias worldwide. It’s particularly useful for identifying related substances closely associated with the main drug. However, it’s only a semi-quantitative method and sensitive to operating conditions, making rapid analysis challenging[6].
- High-Performance Liquid Chromatography (HPLC): HPLC is a mature method for impurity detection in drugs, known for its high separation efficiency, specificity, sensitive detection (down to 0.1% or lower), accuracy, precision, and repeatability.[7] Different types of chromatography can be selected based on the analysis object (normal phase, reverse phase, distribution, etc.). However, HPLC has limitations, such as the need for proper ventilation and a sensitive detector, and challenges when analyzing alkaline drugs or strongly retained substances with long retention times.
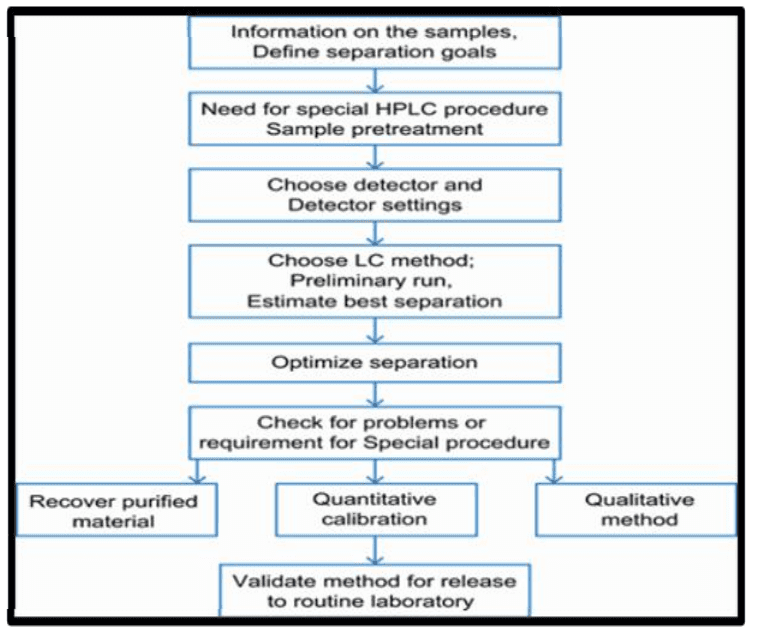
Figure 3 Steps involved in HPLC Method development
- Gas Chromatography (GC): GC is primarily used for volatile organic impurities and is effective for checking residual solvents, especially in Chinese medicine. Examples include determining ethylene oxide residues in metronidazole and various solvent residues in praziquantel.
- Other Chromatography Methods: There are various other chromatography methods, such as Micellar Electrokinetic Chromatography (MEKC) and Ion Chromatography (IC).
Moreover, a multi-method approach is increasingly applied to drug impurity inspection. As analytical techniques advance, the detection and identification of drug impurities improve, aiding in the assessment of drug toxicity, side effects, and prevention of adverse reactions. Reducing impurity content and enhancing drug effectiveness are also vital aspects of impurity control.
Summary
Medications are unique products with a direct impact on our well-being and health. The presence of impurities in drugs significantly influences their quality. Hence, it is imperative to diligently identify the sources, characteristics, detection methods, and allowable limits of drug impurities. Furthermore, ongoing enhancements in drug manufacturing conditions aim to minimize impurity levels. Simultaneously, regulations and standards are developed and enforced to ensure and enhance drug quality comprehensively. These measures collectively work to reduce the incidence of adverse drug reactions.
Reference
- Firouzabadi, H., Iranpoor, N., & Farahi, S. (2008). Solid trichlorotitanium (IV) trifluoromethanesulfonate TiCl3 (OTf) catalyzed efficient acylation of–OH and–SH: Direct esterification of alcohols with carboxylic acids and transesterification of alcohols with esters under neat conditions. Journal of Molecular Catalysis A: Chemical, 289(1-2), 61-68.
- Bau, R., & Tsyba, I. (1999). Crystal structure of amikacin. Tetrahedron, 55(52), 14839-14846.
- Crossman, A. R., Mitchell, I. J., & Sambrook, M. A. (1985). Regional brain uptake of 2-deoxyglucose in N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)—induced parkinsonism in the macaque monkey. Neuropharmacology, 24(6), 587-591.
- Holzgrabe, U., Nap, C. J., Beyer, T., & Almeling, S. (2010). Alternatives to amino acid analysis for the purity control of pharmaceutical grade L‐alanine. Journal of separation science, 33(16), 2402-2410.
- Prince, S. J., McLaury, H. J., Allen, L. V., & McLaury, P. (1999). Analysis of benzalkonium chloride and its homologs: HPLC versus HPCE. Journal of pharmaceutical and biomedical analysis, 19(6), 877-882.
- Poole, C. F. (2003). Thin-layer chromatography: challenges and opportunities. Journal of Chromatography A, 1000(1-2), 963-984.
- Sadapha, P., & Dhamak, K. (2022). Review Article on High-Performance Liquid Chromatography (HPLC) Method Development and Validation. Int. J. Pharm. Sci. Rev. Res, 74, 23-29.