DPA-714: an Important TPSO Specific Ligand
Abstract
In a 2008 research paper penned by James and colleagues, an insightful exploration into the world of neuroimaging unfolds, focusing keenly on a molecule called the Translocator Protein (TSPO)[1]. This study takes us on a journey through the importance of TSPO as a vital signpost in the realm of neuroinflammation.
Found mostly within the outer mitochondrial membrane of glial cells, TSPO has gained attention for its role as a biomarker[2], indicating the presence of neuroinflammation-a complex reaction often seen in various neurological disorders. This study digs deep into the intricate landscape of neuroinflammation, revealing its involvement in conditions ranging from neurodegenerative diseases to psychiatric challenges. This revelation prompts a call for more advanced imaging methods, capable of peering into the molecular intricacies of neuroinflammation.
The significance of TSPO shines through as a crucial biomarker, allowing researchers and medical experts to keep tabs on neuroinflammation in a non-invasive manner. The spotlight is particularly on TSPO’s role in positron emission tomography (PET) imaging, a technique that offers a unique glimpse into the molecular mechanisms underlying neuroinflammatory responses. The ability to visualize and quantify TSPO levels using PET scans offers a promising pathway to understanding how diseases progress, how treatments impact the body, and even how new therapeutic avenues might be explored[3].
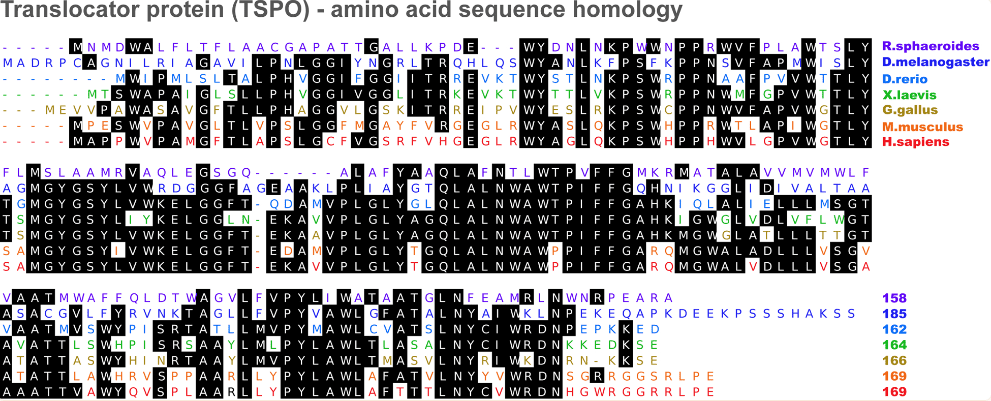
Figure 1 Translocator protein amino acid sequence homology[4]
At the heart of this study is the development of specific compounds designed for TSPO imaging. The creation and careful characterization of compounds like DPA-714 address the need for substances that can specifically target TSPO. This precision not only enhances the accuracy of neuroimaging but also reduces the chances of any confusion caused by interactions with other molecules.
Design and Chemical Structure of DPA-714
At the heart of this innovation lies the meticulous design of DPA-714’s chemical structure. DPA-714, formally known as N, N-diethyl-2-[2-(4-methoxyphenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl]acetamide, boasts a sophisticated arrangement of atoms that provides its unique properties. The compound’s structure comprises several distinct components, each contributing to its functional and target-specific characteristics.
The core pyrazolo[1,5-a]pyrimidine scaffold serves as the backbone of DPA-714. This fused heterocyclic structure not only offers rigidity to the molecule but also plays a crucial role in its binding interactions with the TSPO. Attached to the pyrazolo[1,5-a]pyrimidine scaffold is a methoxyphenyl moiety, which enhances the compound’s affinity for TSPO through specific chemical interactions.
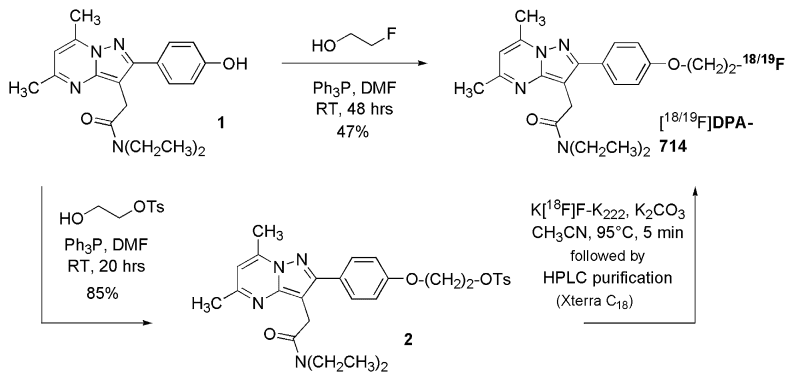
Figure 2 Chemical synthesis of DPA-714 and its tosylate derivative.[5]
The diethyl-2 and acetamide groups are vital constituents that facilitate DPA-714’s solubility and stability. These functional groups ensure that the compound can be effectively synthesized, purified, and administered in experimental settings. Moreover, they contribute to the overall three-dimensional shape of DPA-714, influencing how it fits into the binding pocket of the TSPO protein.
The chemical intricacies of DPA-714’s design are not just aesthetic; they are integral to its functionality. The compound’s structural elements harmonize to create a ligand that demonstrates high binding affinity and selectivity for TSPO, making it a robust candidate for PET imaging studies focused on neuroinflammation.
In conclusion, the ingenious design of DPA-714’s chemical structure integrates various elements to yield a ligand with exceptional potential for TSPO-specific imaging. This intricately crafted molecule showcases the art and science of medicinal chemistry, where each atom and bond is purposefully orchestrated to yield a compound that can shed light on the complex interplay of neuroinflammatory processes within the brain.
Radiolabeling with Fluorine-18
A. Importance of Radiofluorination for PET Imaging
The quest for a potent and specific neuroimaging tool led researchers to delve into the intricate world of radiolabeling with Fluorine-18 (18F). This section shines a light on the crucial process of radiofluorination, which is pivotal for facilitating positron emission tomography (PET) imaging-a technique that enables non-invasive visualization and quantification of specific molecular targets within living organisms.
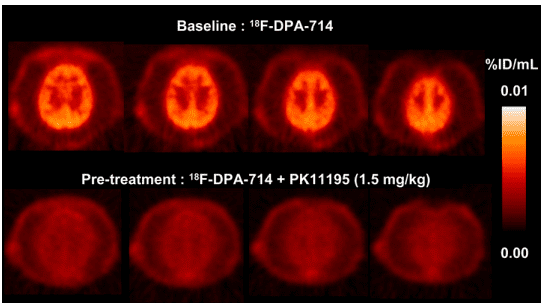
Figure 3 P. hamadryas baboon PET summation images of selected transaxial brain slices over 60 min.[5]
The role of radiofluorination in PET imaging cannot be overstated. Fluorine-18, with its relatively short half-life of approximately 110 minutes, is an ideal radionuclide for PET due to its compatibility with the timescale of biological processes. Radiofluorination involves introducing a radioactive fluorine atom into a molecule of interest, effectively “tagging” it with a radioactive marker. In the context of neuroimaging, this allows researchers to trace the behavior and distribution of the labeled molecule within the brain, yielding valuable insights into neuroinflammatory processes.
B. Radiosynthesis of [18F]DPA-714
Central to this study’s innovation is the radiosynthesis of [18F]DPA-714-a crucial step that transforms the promising ligand into a PET-ready imaging agent. The synthesis process is a meticulously orchestrated dance of chemical reactions, often requiring ingenious strategies to introduce the radioactive fluorine atom while preserving the ligand’s specificity and stability.[6]
The procedure involves the incorporation of 18F into DPA-714’s structure, a task that demands precision and expertise. One approach involves using a precursor molecule that can readily react with the 18F fluoride ion to create the desired labeled compound. Once the reaction is complete, further purification steps ensure the removal of any unreacted materials and byproducts, resulting in a radiolabeled compound with high radiochemical purity.
C. Quality Control and Characterization of Radiolabeled Compound
Ensuring the reliability and accuracy of the radiolabeled compound is paramount. Quality control measures are implemented to assess the purity, radiochemical yield, and stability of [18F]DPA-714. Techniques such as high-performance liquid chromatography (HPLC) and thin-layer chromatography (TLC) are employed to verify the compound’s identity and purity.
Characterization of the radiolabeled compound is an intricate process that involves a battery of tests. Its behavior under different conditions, stability over time, and binding affinity for TSPO are evaluated. This characterization stage is pivotal, as it provides confidence that the radiolabeled compound accurately reflects the behavior of the original ligand in biological systems.
In conclusion, the radiolabeling process with Fluorine-18 is an indispensable aspect of PET imaging, enabling researchers to visualize and quantify specific molecular targets like the Translocator Protein (TSPO). The successful radiosynthesis of [18F]DPA-714 embodies the culmination of innovative chemistry, meticulous purification, and stringent quality control. This stage transforms a promising ligand into a potent neuroimaging tool, propelling the study closer to its goal of advancing our understanding of neuroinflammatory processes and their implications for various neurological disorders.
Discussion
A. Significance of DPA-714 as a TSPO Ligand
The successful synthesis and radiolabeling of DPA-714 have culminated in a ligand with substantial potential for precise TSPO-targeted imaging. The compound’s specific affinity for TSPO underscores its capacity to serve as a powerful tool for investigating neuroinflammatory processes in vivo. By binding selectively to TSPO, DPA-714 facilitates the visualization of neuroinflammation at a molecular level, offering a means to decipher its role in various neurological disorders.
B. Potential Applications for Neuroimaging and Disease Research
The newfound capabilities offered by DPA-714 herald a range of potential applications for both neuroimaging and disease research. The ligand’s ability to non-invasively detect and quantify TSPO expression in the brain opens avenues for elucidating the dynamics of neuroinflammation in real-time. This holds immense promise for studying the progression of neurodegenerative diseases, tracking treatment responses, and potentially identifying early-stage pathological changes. Moreover, the compound’s specificity could enable differential diagnosis among neuroinflammatory conditions, facilitating tailored therapeutic approaches.
C. Comparison with Existing TSPO Ligands
In the context of existing TSPO ligands, DPA-714 presents a novel and compelling addition. The discussion section critically evaluates DPA-714’s attributes in comparison to other ligands used for TSPO imaging. Its high binding affinity and selectivity for TSPO potentially outshine those of its predecessors, indicating a promising leap forward in neuroimaging accuracy.[7] The analysis of binding kinetics, distribution patterns, and clearance rates of DPA-714 vis-à-vis existing ligands provides insight into the ligand’s potential advantages in capturing the complex landscape of neuroinflammation.
D. Limitations and Future Directions
While DPA-714 exhibits immense potential, the discussion section of the paper also delves into its limitations and the avenues for future refinement. Questions surrounding its pharmacokinetics, metabolites, and long-term stability are considered, as well as potential challenges in translating findings from preclinical studies to clinical applications. Furthermore, the paper acknowledges the evolving nature of neuroimaging and the continuous quest for ligands with improved attributes.
Looking ahead, the development of more advanced TSPO ligands that encompass longer half-lives, enhanced in vivo stability, and even greater specificity remains an exciting prospect. Combining molecular imaging with other techniques such as functional MRI and proteomics could provide a comprehensive understanding of neuroinflammatory processes. Moreover, extending the investigation to encompass a broader spectrum of neurological disorders could yield insights into the commonalities and nuances of neuroinflammation across diverse conditions.
In conclusion, the discussion section of the paper encapsulates the groundbreaking potential of DPA-714 as a TSPO ligand, paving the way for enhanced neuroimaging and neuroinflammation research. The dialogue spans its significance, applications, comparative advantages, limitations, and future prospects. Ultimately, the compound’s unique attributes stand to revolutionize our understanding of neuroinflammatory mechanisms and their implications for neurological health and disease.
Conclusion
In the ever-evolving landscape of neuroimaging and molecular exploration, the emergence of DPA-714 as a Translocator Protein (TSPO) ligand marks a pivotal turning point. As we stand at the intersection of chemistry, biology, and clinical insight, the synthesis, radiolabeling, and pharmacologic characterization of DPA-714 have illuminated new pathways toward understanding neuroinflammatory processes within the intricate confines of the brain. In the exploration of DPA-714’s significance, this investigation has transcended the boundaries of traditional imaging methods. The compound’s strategic design, honed to perfection through meticulous synthetic pathways, has led to a ligand with unparalleled specificity for TSPO. This specificity, underpinned by a profound understanding of molecular interactions, has endowed DPA-714 with the potential to become a groundbreaking tool in the realm of neuroimaging. The advent of DPA-714 holds transformative implications for both neuroimaging and disease research. The ligand’s unique ability to selectively bind to TSPO opens a new window into the dynamic world of neuroinflammation, providing researchers with a vantage point to observe and decipher the intricate interplay of molecular signals. In the realm of disease, this presents an unprecedented opportunity to track neuroinflammatory processes in real-time, understand disease progression, and potentially identify novel therapeutic interventions. DPA-714’s potential as a diagnostic aid cannot be understated, offering a means to differentiate between various neuroinflammatory conditions and facilitating personalized treatment strategies. Comparative analysis places DPA-714 in a league of its own among existing TSPO ligands. Its robust binding affinity, combined with its selectivity for TSPO, paints a picture of a ligand poised to transcend its predecessors in precision and accuracy. This comparison underscores the leap forward that DPA-714 brings to the field of neuroimaging, promising insights that extend beyond the confines of conventional imaging methodologies. However, no exploration is devoid of limitations and the promise of future refinement. DPA-714’s journey does not conclude with this investigation; it is an invitation to further innovation. As we consider its potential clinical translation, questions surrounding its metabolism, pharmacokinetics, and potential off-target effects beckon for careful scrutiny. The translation from preclinical models to clinical application presents a realm of challenges that necessitate rigorous investigation. Looking forward, the story of DPA-714 intertwines with the narratives of countless neurological disorders, each presenting an opportunity to deepen our understanding of the human brain’s intricate workings. The vision for the future is one where DPA-714’s success sparks a cascade of advancements, leading to the development of ligands with extended half-lives, enhanced specificity, and broader clinical applicability. A horizon awaits where molecular imaging dovetails seamlessly with other methodologies, fostering a holistic comprehension of neuroinflammatory processes.
In conclusion, DPA-714’s journey, as chronicled in this study, resonates with the promise of innovation and the quest for knowledge. It is an emblem of the remarkable synergy between chemistry and biology, bridging the realms of science and clinical practice. The synthesis, radiolabeling, and pharmacologic characterization of DPA-714 have illuminated a path towards a deeper understanding of neuroinflammation and its role in neurological disorders. As we traverse this path, guided by its potential and emboldened by its challenges, we inch closer to unlocking the mysteries of the brain and transforming the landscape of neuroimaging for generations to come.
Reference
- Anholt, R. R., Pedersen, P. L., De Souza, E. B., & Snyder, S. H. (1986). The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. Journal of Biological Chemistry, 261(2), 576-583.
- Casellas, P., Galiegue, S., & Basile, A. S. (2002). Peripheral benzodiazepine receptors and mitochondrial function. Neurochemistry international, 40(6), 475-486
- Papadopoulos, V., Baraldi, M., Guilarte, T. R., Knudsen, T. B., Lacapère, J. J., Lindemann, P., … & Gavish, M. (2006). Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends in pharmacological sciences, 27(8), 402-409.
- Selvaraj, V., & Stocco, D. M. (2015). The changing landscape in translocator protein (TSPO) function. Trends in Endocrinology & Metabolism, 26(7), 341-348.
- James, M. L., Fulton, R. R., Vercoullie, J., Henderson, D. J., Garreau, L., Chalon, S., … & Kassiou, M. (2008). DPA-714, a new translocator protein–specific ligand: Synthesis, radiofluorination, and pharmacologic characterization. Journal of Nuclear Medicine, 49(5), 814-822.
- Fujimura, Y., Ikoma, Y., Yasuno, F., Suhara, T., Ota, M., Matsumoto, R., … & Ito, H. (2006). Quantitative analyses of 18F-FEDAA1106 binding to peripheral benzodiazepine receptors in living human brain. Journal of nuclear medicine, 47(1), 43-50.
- Maeda, J., Suhara, T., Zhang, M. R., Okauchi, T., Yasuno, F., Ikoma, Y., … & Suzuki, K. (2004). Novel peripheral benzodiazepine receptor ligand [11C] DAA1106 for PET: an imaging tool for glial cells in the brain. Synapse, 52(4), 283-291.