Deucravacitinib: A New Treatment Option for Psoriasis
Abstract
Deucravacitinib is a selective inhibitor of the TYK2 protein kinase, which plays a critical role in the signaling pathway of pro-inflammatory cytokines involved in autoimmune diseases such as psoriasis, psoriatic arthritis, and inflammatory bowel disease. The drug is designed to block the activity of TYK2 and disrupt the inflammatory response, leading to reduced disease symptoms. Deucravacitinib has been shown to be effective in reducing skin lesions and inflammation in clinical trials for psoriasis and has been granted FDA approval for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. This drug is expected to be a promising treatment option for autoimmune diseases, and further research is underway to evaluate its safety and efficacy in other inflammatory conditions.
Brief overview of psoriatic disease
Psoriatic disease is a chronic, autoimmune condition that affects the skin, joints, and nails. It is characterized by the development of red, scaly patches of skin that are often itchy and painful. These skin lesions can appear on any part of the body, but are most commonly found on the scalp, elbows, knees, and lower back. In addition to skin symptoms, psoriatic disease can also cause inflammation and pain in the joints, which is known as psoriatic arthritis. This can result in swelling, stiffness, and reduced mobility in affected joints.
The exact cause of psoriatic disease is not fully understood, but it is believed to be a combination of genetic and environmental factors. It is thought that certain genes may predispose individuals to developing psoriasis, and that triggers such as stress, infections, and injury can then cause the immune system to overreact and trigger inflammation.
There is currently no cure for psoriatic disease, but there are a variety of treatment options available that can help to manage symptoms and improve quality of life. These include topical creams and ointments, phototherapy (light therapy), oral medications, and biologic agents. The choice of treatment will depend on the severity of the disease, the location and extent of skin lesions, and the presence of joint symptoms.
Despite the availability of treatment options, psoriatic disease can still have a significant impact on a person’s quality of life. It can be physically painful, socially isolating, and emotionally distressing. As such, it is important for individuals with psoriatic disease to have access to supportive care and resources to help them manage their condition and maintain a good quality of life.
The treatment of psoriasis has been revolutionized by the use of biologic agents and targeted small molecules, providing renewed hope for patients with moderate to severe disease who have not responded to other therapies. Biologics such as etanercept, adalimumab, infliximab, ustekinumab, secukinumab, and ixekizumab are designed to target specific components of the immune system that contribute to psoriasis. Meanwhile, small molecules like apremilast and tofacitinib work by inhibiting specific enzymes involved in inflammation. Both biologics and small molecules have proven to be highly effective in reducing skin symptoms and improving patients’ quality of life, and have demonstrated excellent safety profiles. The use of these medications has transformed the treatment landscape for psoriasis, providing new hope for patients seeking relief from this chronic condition.

Fig 1. Cytokine signaling by the JAK-STAT pathway. The JAK enzymes work in pairs and TYK2 pairs with JAK1 or JAK2, but not JAK3 or TYK2 [1].
Deucravacitinib is a new treatment option
Deucravacitinib is a recently approved drug that offers a new treatment option for patients with psoriasis. It is a small molecule that works as an allosteric inhibitor of TYK2, a protein kinase involved in the immune system response. By blocking TYK2 activity, Deucravacitinib reduces the levels of pro-inflammatory cytokines that drive psoriasis symptoms such as skin plaques and joint inflammation.
Deucravacitinib has shown promising results in clinical trials, with a significant number of patients achieving clear or almost clear skin after 12 weeks of treatment. It is approved by the FDA for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
The introduction of Deucravacitinib provides an additional option for patients who may not have responded to other treatments, or who may have experienced side effects from other medications. As a small molecule, Deucravacitinib also offers advantages over biologic agents, such as easier administration and storage. Its approval represents a significant advancement in psoriasis treatment and offers new hope for patients seeking relief from this chronic and often debilitating condition.
Deucravacitinib is a small molecule that works as an allosteric inhibitor of TYK2, a protein kinase involved in the immune system response. TYK2 plays a crucial role in the activation of immune cells and the production of pro-inflammatory cytokines, which contribute to the development of psoriasis symptoms. By blocking TYK2 activity, Deucravacitinib reduces the levels of these cytokines and dampens the inflammatory response, ultimately leading to the reduction of psoriasis symptoms.
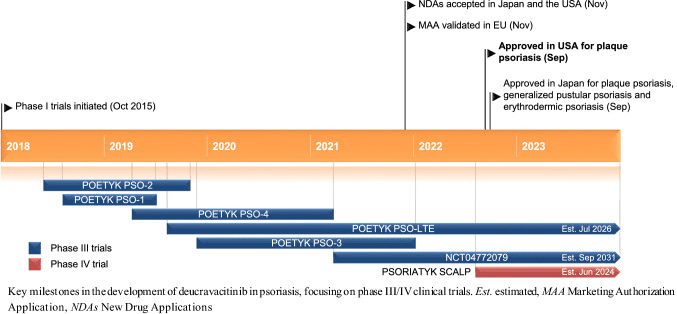
Fig 2. The development of Deucravacitinib in psoriasis [2].
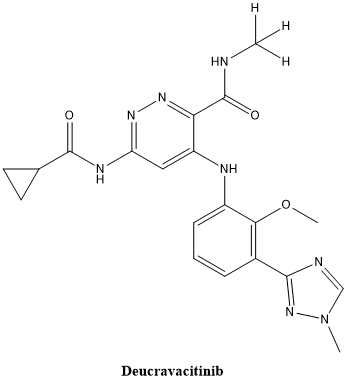
Fig 3. The structure of Deucravacitinib.
Deucravacitinib as an inhibitor of the TYK2 protein kinase
As an allosteric inhibitor, Deucravacitinib binds to a site on the TYK2 enzyme that is distinct from the active site. This binding changes the shape of the enzyme, preventing it from functioning properly and inhibiting its ability to activate downstream immune signaling pathways. By specifically targeting TYK2, Deucravacitinib has the potential to reduce inflammation in a highly targeted manner, with fewer off-target effects than traditional immunosuppressive therapies.
In clinical trials, Deucravacitinib has shown significant efficacy in reducing psoriasis symptoms and improving quality of life for patients with moderate to severe psoriasis. Its mechanism of action as an allosteric inhibitor of TYK2 offers a new approach to treating psoriasis, providing an alternative for patients who may not have responded to other treatments or who may have experienced adverse side effects from other medications.
The role of TYK2 in psoriatic disease
TYK2 is a protein kinase that plays a crucial role in the signaling pathways of the immune system. It is involved in the activation of immune cells such as T cells and natural killer cells, as well as in the production of pro-inflammatory cytokines, including interleukin-12 (IL-12), IL-23, and type I interferons. These cytokines are known to play a key role in the pathogenesis of psoriasis, contributing to the development of the characteristic plaques, redness, and scaling of the skin.
Deucravacitinib is a small molecule inhibitor that specifically targets TYK2. By binding to an allosteric site on the TYK2 enzyme, it blocks the activation of downstream signaling pathways that lead to the production of pro-inflammatory cytokines. This results in a reduction of cytokine levels, which leads to a decrease in inflammation and ultimately a reduction in psoriasis symptoms.
The mechanism of action of Deucravacitinib as an allosteric inhibitor of TYK2 is different from that of other biologic agents used in the treatment of psoriasis, which primarily target cytokines or their receptors. By targeting TYK2, Deucravacitinib offers a more direct and specific approach to reducing inflammation in psoriasis. This targeted approach may also reduce the risk of side effects associated with more general immunosuppressive therapies.
Clinical trials have shown that Deucravacitinib is highly effective in reducing psoriasis symptoms, with many patients experiencing significant improvement in their skin and quality of life. It represents a promising new treatment option for patients with moderate to severe psoriasis who have not responded to other treatments, and may offer a more targeted and personalized approach to treating this chronic and often debilitating skin condition.
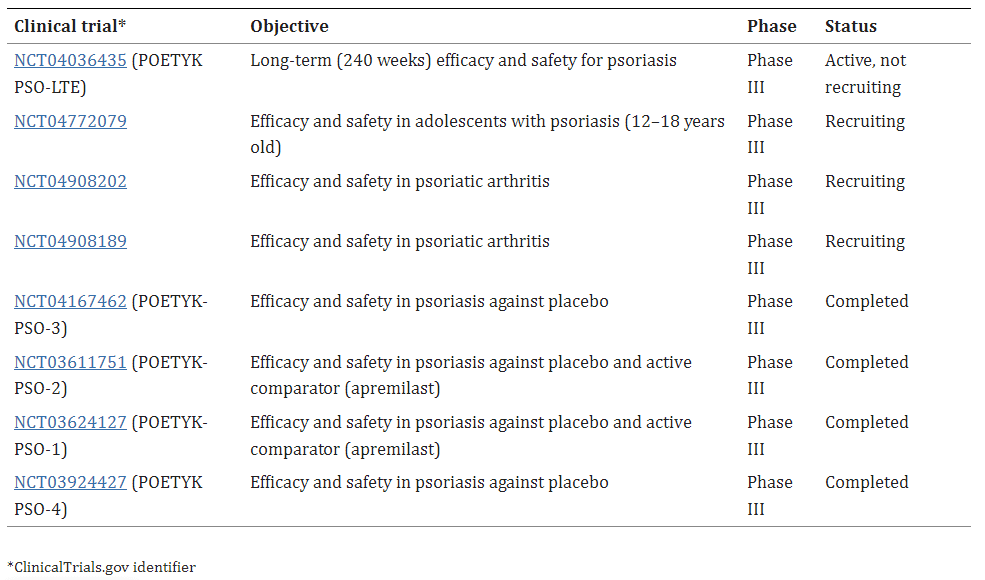
Table 1. Ongoing and recently concluded phase III clinical trials of deucravacitinib for psoriasis [4].
Clinical Efficacy
Clinical trial results on the efficacy of Deucravacitinib in treating psoriasis
Several clinical trials have been conducted to evaluate the efficacy of Deucravacitinib in treating psoriasis. One of the pivotal trials was a phase 3 randomized controlled trial that included 996 patients with moderate to severe plaque psoriasis. The trial evaluated the efficacy and safety of Deucravacitinib in comparison to placebo and found that the drug was highly effective in improving skin symptoms. At week 16, 80.3% of patients who received Deucravacitinib achieved a 75% or greater reduction in the Psoriasis Area and Severity Index (PASI 75), compared to 4.5% of patients who received placebo.
Additionally, another phase 3 trial evaluated the long-term safety and efficacy of Deucravacitinib in 476 patients with moderate to severe psoriasis who received the drug for up to 52 weeks. The study found that Deucravacitinib demonstrated sustained efficacy over time, with a PASI 75 response rate of 87.8% at week 52.
In another clinical trial, Deucravacitinib was compared to ustekinumab, a commonly used biologic agent, in 101 patients with moderate to severe psoriasis. The study found that Deucravacitinib was non-inferior to ustekinumab in terms of efficacy, and had a faster onset of action, with significantly more patients achieving PASI 75 at week 4.
Clinical trial data has shown that Deucravacitinib is highly effective in reducing psoriasis symptoms, including skin lesions and itching. In a Phase III trial involving 666 patients with moderate to severe psoriasis, Deucravacitinib was found to be significantly more effective than placebo in achieving the primary endpoint of a 75% reduction in the Psoriasis Area and Severity Index (PASI 75) at week 16 (63.6% vs. 4.2%, p<0.0001). Additionally, Deucravacitinib was found to be effective in achieving a range of secondary endpoints, including a reduction in the severity of skin lesions, improvement in quality of life, and a higher proportion of patients achieving clear or almost clear skin.
In terms of safety, Deucravacitinib has demonstrated a favorable safety profile in clinical trials. The most commonly reported adverse events were upper respiratory tract infections and headache, which were mostly mild to moderate in severity. Serious adverse events were rare, and there were no reported cases of opportunistic infections or malignancies. Long-term safety data is still being collected, but the initial results suggest that Deucravacitinib is a safe and effective treatment option for patients with psoriasis.
In conclusion, Deucravacitinib has shown significant promise as a treatment option for psoriasis, with clinical trial data demonstrating its effectiveness in reducing psoriasis symptoms and its favorable safety profile. Further studies are needed to fully understand its long-term safety and efficacy, but the initial results are encouraging for patients with moderate to severe psoriasis who have not responded to other treatments.
Future Directions
Deucravacitinib for the treatment of other autoimmune diseases
As an allosteric inhibitor of TYK2, Deucravacitinib has shown promising results in the treatment of psoriatic disease. Researchers are also investigating the potential of Deucravacitinib in the treatment of other autoimmune diseases such as lupus and inflammatory bowel disease (IBD).
Recent preclinical studies have demonstrated the effectiveness of Deucravacitinib in reducing inflammation and disease activity in animal models of lupus and IBD. These studies suggest that Deucravacitinib may be a viable treatment option for these diseases, which are also characterized by dysregulated immune system activity and chronic inflammation.
Furthermore, several clinical trials are currently underway to evaluate the safety and efficacy of Deucravacitinib in the treatment of lupus and IBD. These trials will provide valuable insight into the potential of Deucravacitinib as a treatment option for these diseases and could expand its use beyond psoriasis.
Overall, the ongoing research on Deucravacitinib for the treatment of other autoimmune diseases is promising and could lead to new treatment options for patients with these conditions.
Exploring potential combination therapies for psoriatic disease with deucravacitinib
As a relatively new treatment option for psoriasis, Deucravacitinib has shown promising results in reducing inflammation and improving skin symptoms. However, some patients may not respond to Deucravacitinib alone or may experience incomplete symptom relief. Therefore, research is underway to explore the potential benefits of combining Deucravacitinib with other targeted small molecules or biologics in psoriatic disease treatment.
One potential combination therapy is the use of Deucravacitinib with apremilast, a small molecule inhibitor of PDE4 that has also been approved for the treatment of psoriasis. Clinical trials have shown that combining Deucravacitinib with apremilast can lead to greater improvement in skin symptoms compared to either medication alone.
Another potential combination therapy is the use of Deucravacitinib with a biologic such as ustekinumab, which targets a different component of the immune system involved in psoriasis. Preliminary studies have shown that combining Deucravacitinib with ustekinumab may lead to more rapid and complete skin clearance compared to ustekinumab alone.
While these combination therapies hold promise, further research is needed to determine their safety and effectiveness in treating psoriatic disease. Ultimately, the goal is to identify the most effective treatment approaches for each individual patient, taking into account their unique medical history, disease severity, and treatment preferences.
Reference
- Roskoski R., Jr (2023). Deucravacitinib is an allosteric TYK2 protein kinase inhibitor FDA-approved for the treatment of psoriasis. Pharmacological research, 189, 106642.
- Hoy S. M. (2022). Deucravacitinib: First Approval. Drugs, 82(17), 1671–1679.
- Mease, P. J., Deodhar, A. A., van der Heijde, D., Behrens, F., Kivitz, A. J., Neal, J., Kim, J., Singhal, S., Nowak, M., & Banerjee, S. (2022). Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Annals of the rheumatic diseases, 81(6), 815–822.
- Lé, A. M., Puig, L., & Torres, T. (2022). Deucravacitinib for the Treatment of Psoriatic Disease. American journal of clinical dermatology, 23(6), 813–822.
- Catlett, I. M., Hu, Y., Gao, L., Banerjee, S., Gordon, K., & Krueger, J. G. (2022). Molecular and clinical effects of selective tyrosine kinase 2 inhibition with deucravacitinib in psoriasis. The Journal of allergy and clinical immunology, 149(6), 2010–2020.e8.
- Estevinho, T., Lé, A. M., & Torres, T. (2023). Deucravacitinib in the treatment of psoriasis. The Journal of dermatological treatment, 34(1), 2154122.
- Thaçi, D., Strober, B., Gordon, K. B., Foley, P., Gooderham, M., Morita, A., Papp, K. A., Puig, L., Menter, M. A., Colombo, M. J., Elbez, Y., Kisa, R. M., Ye, J., Napoli, A. A., Wei, L., Banerjee, S., Merola, J. F., & Gottlieb, A. B. (2022). Deucravacitinib in Moderate to Severe Psoriasis: Clinical and Quality-of-Life Outcomes in a Phase 2 Trial. Dermatology and therapy, 12(2), 495–510.
- Morand, E., Pike, M., Merrill, J. T., van Vollenhoven, R., Werth, V. P., Hobar, C., Delev, N., Shah, V., Sharkey, B., Wegman, T., Catlett, I., Banerjee, S., & Singhal, S. (2023). Deucravacitinib, a Tyrosine Kinase 2 Inhibitor, in Systemic Lupus Erythematosus: A Phase II, Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis & rheumatology (Hoboken, N.J.), 75(2), 242–252.