Degrader-Antibody Conjugates (DACs): The Future of Targeted Cancer Therapy
Abstract
Degrader-Antibody Conjugates (DACs) are a cutting-edge class of therapeutics that merge the selectivity of monoclonal antibodies with the catalytic efficiency of targeted protein degraders, such as PROTACs and molecular glues. This blog post explores the foundational principles, mechanism of action, therapeutic applications, development challenges, and future market outlook of DACs in modern drug discovery. As a next-generation alternative to traditional Antibody-Drug Conjugates (ADCs), DACs show immense promise in treating previously undruggable cancers and other complex diseases. With biotech and pharmaceutical companies rapidly advancing DAC platforms, these conjugates are poised to redefine targeted therapy in precision medicine.
What Are Degrader-Antibody Conjugates? The Next Evolution in Targeted Therapy
Degrader-Antibody Conjugates (DACs) are an emerging class of biologic therapeutics that combine the precision of monoclonal antibodies with the powerful mechanism of targeted protein degradation. As a next-generation evolution of Antibody-Drug Conjugates (ADCs), DACs go beyond inhibition — they eliminate disease-causing proteins from within cells. This innovative approach holds significant promise for treating cancer and other diseases where conventional inhibitors have failed.
In DACs, a monoclonal antibody is chemically linked to a protein degrader, such as a PROTAC (PROteolysis TArgeting Chimera) or a molecular glue. The antibody selectively binds to a tumor-specific antigen on the cell surface, facilitating the internalization of the complex. Once inside the cell, the degrader component hijacks the cell’s ubiquitin-proteasome system, recruiting an E3 ligase to tag the target protein for destruction. This catalytic mechanism enables potent and sustained suppression of oncogenic proteins.
What sets DACs apart is their ability to tackle previously “undruggable” intracellular targets. Unlike ADCs that deliver cytotoxic payloads, DACs offer a more refined, mechanism-driven approach that could reduce systemic toxicity while increasing therapeutic efficacy. Recent preclinical studies and early-phase clinical trials have already shown encouraging results in solid tumors and hematologic malignancies.
With biotech companies and academic researchers investing heavily in DAC development, this novel modality is poised to redefine the landscape of targeted therapy in oncology and beyond.
How Degrader-Antibody Conjugates Work: A Mechanistic Deep Dive
Degrader-Antibody Conjugates (DACs) are a novel class of targeted therapies designed to harness the body’s own protein degradation machinery. At their core, DACs integrate two key elements: the targeting specificity of monoclonal antibodies and the protein-eliminating power of degraders such as PROTACs (PROteolysis TArgeting Chimeras) or molecular glues.
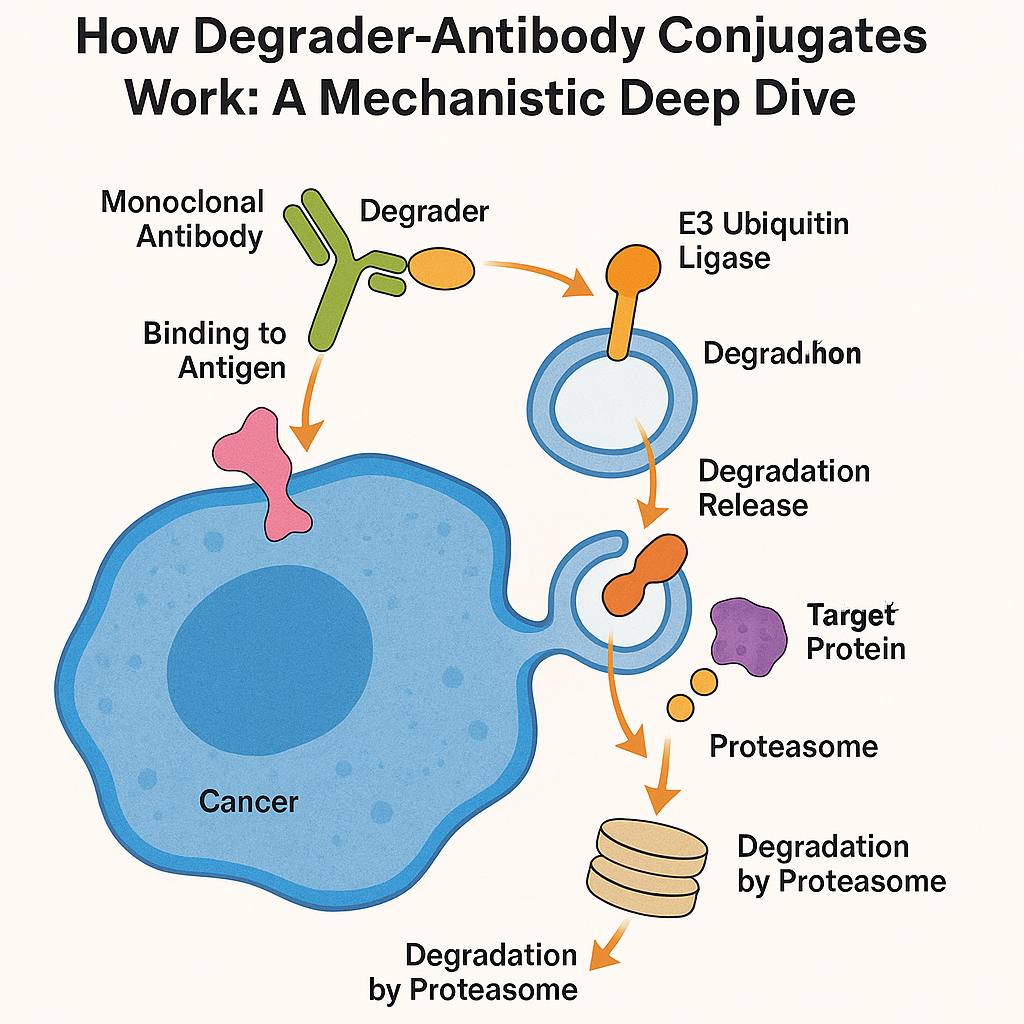
The mechanism of action begins when the antibody component of a DAC binds to a specific antigen on the surface of a cancer cell—examples include Trop2, CD123, or ROR1. This targeting ensures that the payload reaches only the intended diseased cells, minimizing off-target toxicity. Once bound, the entire antibody-degrader complex is internalized through receptor-mediated endocytosis.
Inside the cell, the degrader payload is released and becomes active. PROTACs and molecular glues operate by recruiting E3 ubiquitin ligases (such as CRBN or VHL) to the target protein. This proximity triggers the transfer of ubiquitin molecules onto the target, marking it for destruction by the proteasome—effectively removing the protein from the cell in a catalytic, repeatable process.
Unlike traditional inhibitors that merely block protein activity, DACs physically eliminate the target proteins, enabling longer-lasting and often more complete biological responses. Moreover, DACs can access intracellular targets once considered “undruggable,” such as transcription factors or scaffold proteins.
This highly specific and catalytic degradation mechanism is what makes DACs a promising platform for future cancer therapies—particularly in tumors that have developed resistance to conventional small molecules or ADCs.
Therapeutic Applications and Advantages of Degrader-Antibody Conjugates (DACs)
Degrader-Antibody Conjugates (DACs) represent a transformative therapeutic platform with broad potential across multiple disease areas, especially in oncology. Their ability to eliminate disease-driving intracellular proteins through targeted degradation offers a new line of attack where traditional drugs often fall short.
The most promising application of DACs is in cancer therapy, particularly for tumors that express unique surface antigens and harbor “undruggable” intracellular oncogenes. For instance, DACs have shown efficacy in preclinical and early clinical trials targeting antigens like ROR1, Trop2, and CD123—which are often overexpressed in hematologic malignancies (e.g., acute myeloid leukemia) and solid tumors (e.g., prostate, breast, and lung cancers).
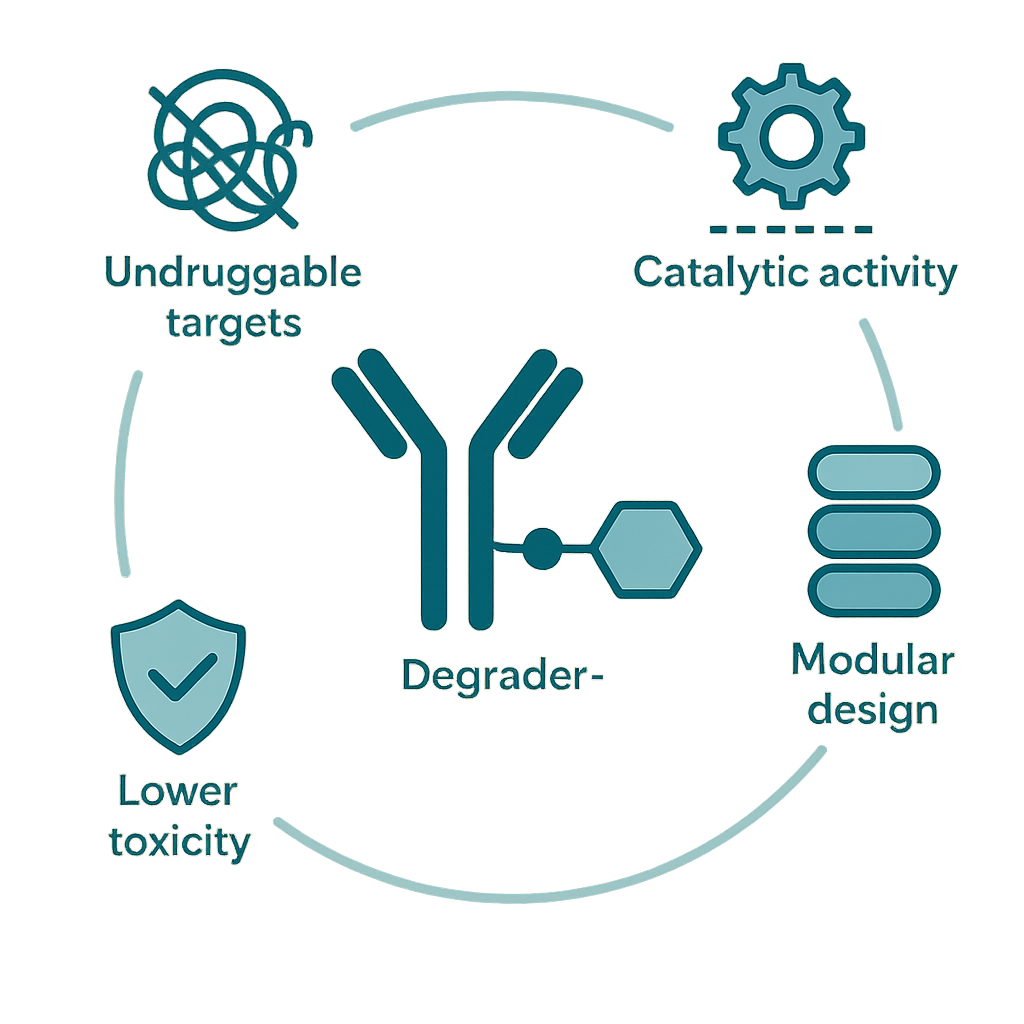
One of the key advantages of DACs is their catalytic mode of action. Unlike inhibitors or cytotoxic drugs that require sustained high concentrations, DACs facilitate protein degradation at substoichiometric doses, leading to durable target knockdown and potentially lower systemic toxicity. Their modular design—antibody, linker, and degrader payload—also allows customization to disease-specific targets and resistance mechanisms.
In addition, DACs can degrade proteins previously considered “undruggable,” such as transcription factors and scaffold proteins, which lack active binding sites. This unlocks new biological targets and therapeutic strategies that were previously out of reach with traditional small molecules or ADCs.
As biotech companies and pharma continue to invest in DAC pipelines, these innovative biologics are expected to play a pivotal role in the next generation of precision medicine—with the potential to revolutionize treatment for refractory and high-mutation-burden cancers.
Challenges in Degrader-Antibody Conjugate (DAC) Development
While Degrader-Antibody Conjugates (DACs) offer groundbreaking potential in targeted protein degradation, several technical and biological challenges must be addressed before widespread clinical adoption becomes a reality.
One of the most significant hurdles lies in payload design and stability. Most DACs use PROTACs or molecular glues as payloads—both of which tend to be large, lipophilic, and chemically complex. These properties can negatively impact solubility, conjugation efficiency, and antibody stability. Maintaining the integrity and potency of the payload throughout manufacturing and systemic circulation remains a key formulation challenge.
Another challenge is linker chemistry. The linker must strike a delicate balance: it must be stable in the bloodstream to prevent premature release, yet cleavable inside the target cell to ensure functional delivery of the degrader. Engineering such linkers requires precise tuning of enzymatic or pH-sensitive mechanisms that can vary across different tissues and tumors.
Intracellular trafficking poses a further obstacle. Once the DAC is internalized via receptor-mediated endocytosis, the degrader payload must escape the endosome and reach the cytoplasm or nucleus to engage its E3 ligase and target protein. Ensuring efficient endosomal escape without degradation or sequestration remains an unsolved issue in many current designs.
Additionally, there are concerns about immunogenicity, biodistribution, and off-target degradation, particularly as DACs enter systemic circulation. Fine-tuning the antibody specificity and optimizing the drug-antibody ratio (DAR) is critical to minimize unintended interactions.
Despite these challenges, ongoing innovation in linker design, computational modeling, and antibody engineering is rapidly pushing the DAC field forward, with many obstacles now being actively addressed by both academia and industry.
Future Trends and Market Outlook for Degrader-Antibody Conjugates (DACs)
The future of Degrader-Antibody Conjugates (DACs) is bright, with increasing investment and innovation pushing this next-generation therapeutic modality closer to clinical reality. As more protein degradation technologies mature, DACs are emerging as a highly adaptable platform capable of reshaping the oncology drug development landscape—and potentially expanding into autoimmune, neurodegenerative, and infectious diseases.
A major future trend is the integration of molecular glue degraders and covalent degraders as DAC payloads. These alternatives to traditional PROTACs offer smaller, more drug-like molecules with improved stability and manufacturability—critical for scale-up and commercial viability. Moreover, innovations in linker technologies are making it easier to develop tunable, tissue-specific DACs with minimal off-target effects.
Another key development is the expansion of target scope. Biotech companies are now designing DACs to degrade transcription factors, scaffolding proteins, and epigenetic regulators—many of which were previously considered undruggable. This opens up new therapeutic options for difficult-to-treat cancers and genetic diseases.
On the business front, several biotech firms—including Crossfire Oncology, Kymera Therapeutics, and Orum Therapeutics—are advancing DAC candidates into preclinical and early-phase clinical trials. Candidates like ORM-5029 and BMS-986497 are among the first DACs showing promise in patients with breast cancer and acute myeloid leukemia.
As AI and structure-based design tools become more advanced, they are also being used to optimize payload-antibody pairings, binding kinetics, and E3 ligase compatibility, making DAC discovery more efficient.
According to industry analysts, the DAC market is projected to follow a growth trajectory similar to ADCs—potentially becoming a multi-billion-dollar segment within the biopharmaceutical industry over the next decade.
References
Wang, L., Ke, Y., He, Q., Paerhati, P., & Zhuang, W. (2025). A novel ROR1-targeting antibody-PROTAC conjugate promotes BRD4 degradation for solid tumor treatment. Frontiers in Oncology.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11729552/
Song, S., Liu, Y., Liu, J., & Tai, W. (2025). In silico-driven THIOMAB approach for stable PROTAC conjugates by docking payloads in antibody cavities. Bioconjugate Chemistry, 36(7), 1445–1456.
https://pubs.acs.org/doi/abs/10.1021/acs.bioconjchem.4c00588
Dong, X., Guo, Y., Zhang, J., Shen, Z., Wu, S., & Ma, X. (2024). Potent in vitro and in vivo efficacy of Hdz-C123A, a GSPT1 degrader-antibody conjugate targeting CD123 in acute myeloid leukemia. Blood, 143(12), 2167–2179.
https://www.sciencedirect.com/science/article/pii/S0006497124029033
Yi, D., Zhang, W., Liu, J., Lee, S., & Chen, A. (2025). A linker platform for antibody drug conjugates (ADCs): Expanding the therapeutic window of dual-payload degrader-antibody conjugates. Cancer Research, 85(8 Suppl), Abstract 7463.
https://aacrjournals.org/cancerres/article/85/8_Supplement_1/7463/759625
Chen, C., Shen, Q., Cai, X., Ding, Y., Yang, X., & Fang, Y. (2025). Discovery and characterization of DAC-1522, a novel Trop2-targeting degrader-antibody conjugate for precision oncology. Cancer Research, 85(8 Suppl), Abstract 7005.
https://aacrjournals.org/cancerres/article/85/8_Supplement_1/7005/759432
Xi, M., Zhang, F., Zhu, J., Shen, H., & Gao, X. (2025). Biomacromolecule-mediated targeted protein degradation: An emerging strategy for cancer therapy. European Journal of Medicinal Chemistry.
https://www.sciencedirect.com/science/article/abs/pii/S0223523425007846



