Decoding Cancer Epigenetics: How YTH Domain Proteins Read m6A to Rewrite Tumor Fate
Abstract
Recent advances in epitranscriptomics have revealed that RNA modifications, particularly N6-methyladenosine (m6A), play crucial roles in regulating gene expression beyond the DNA level. At the heart of this regulation are YTH domain-containing proteins, the primary “readers” of m6A marks. These proteins orchestrate key post-transcriptional processes such as mRNA splicing, translation, export, and degradation. In cancer, the YTH family exhibits diverse and often context-specific functions—some promote tumor growth and metastasis, while others act as tumor suppressors. Structural insights into the YTH domain have opened up new opportunities for the development of targeted cancer therapies, making YTH proteins a promising focus in precision oncology. This article explores the functional mechanisms, structural features, and therapeutic implications of YTH family proteins in cancer biology.
Introduction: m6A – The Epigenetic Switch You Need to Know
In the intricate landscape of gene expression, scientists have long focused on DNA and histone modifications as key epigenetic regulators. However, in recent years, attention has shifted toward the dynamic chemical changes that occur at the RNA level—especially a modification known as N6-methyladenosine (m6A). As the most abundant internal modification of messenger RNA (mRNA) in eukaryotic cells, m6A has emerged as a powerful regulator of RNA metabolism and cellular function.
First discovered in the 1970s, m6A occurs at specific consensus sequences (typically DRACH motifs, where D = A/G/U, R = A/G, H = A/C/U) and is especially enriched in the coding sequence (CDS) and 3′ untranslated region (3′ UTR) of mRNAs. What makes m6A truly fascinating is its reversible nature. Like the methylation marks on DNA, m6A modifications can be written, erased, and read—adding an extra layer of regulatory complexity to the transcriptome.
This regulation is orchestrated by three groups of proteins:
Writers: A methyltransferase complex (e.g., METTL3, METTL14, WTAP) that installs the m6A mark.
Erasers: Demethylases (e.g., FTO, ALKBH5) that remove the mark, reversing the effect.
Readers: RNA-binding proteins that recognize m6A and determine the fate of the modified transcript.
Among these, YTH domain-containing proteins are the most studied class of m6A readers. These proteins do not simply detect m6A—they actively influence critical processes such as mRNA splicing, translation, decay, and export. Their functions are not only essential for normal cellular homeostasis but are now recognized as key players in cancer progression and resistance.
Advances in sequencing technologies like m6A-seq, CLIP-seq, and PAR-CLIP have allowed researchers to map m6A sites transcriptome-wide and dissect how YTH family proteins interact with m6A-modified transcripts. This has propelled the field of epitranscriptomics into the spotlight and opened exciting avenues for cancer diagnostics and therapeutics.
As we continue to decode the molecular grammar of m6A and its readers, one thing becomes clear: epigenetic control doesn’t stop at the genome—it extends deep into the world of RNA.
Meet the Readers: The YTH Family and How They Work
Once m6A is installed on RNA transcripts, it doesn’t act alone—it requires readers to interpret its message. Among these, the YTH (YT521-B homology) domain-containing proteins have emerged as the central readers of m6A modifications. These proteins possess a conserved YTH domain that recognizes m6A-marked RNAs and directs their splicing, transport, translation, or degradation, depending on the cellular context.
The YTH family in humans includes five main proteins: YTHDF1, YTHDF2, YTHDF3, YTHDC1, and YTHDC2. Despite their shared m6A-binding capabilities, each member plays a unique role in RNA metabolism:
YTHDF1 binds m6A-containing mRNAs in the cytoplasm and enhances translation initiation and elongation by recruiting ribosomes and translation initiation factors. It has also been implicated in promoting axon regeneration and learning-related plasticity.
YTHDF2 primarily promotes mRNA decay. It recognizes methylated transcripts and recruits the CCR4-NOT deadenylase complex or engages in HRSP12-mediated cleavage, leading to selective degradation of the RNA. This role is crucial in reducing oncogenic transcript levels in several cancers.
YTHDF3 serves as a versatile coordinator, interacting with YTHDF1 to boost translation or with YTHDF2 to promote decay. Some models propose that YTHDF3 acts as an “assigner”, directing methylated RNAs toward either fate depending on context.
YTHDC1, localized in the nucleus, regulates mRNA splicing by recruiting SRSF3 and blocking SRSF10, and also mediates mRNA export. It plays a critical role in X-chromosome inactivation, chromatin remodeling, and transcriptional repression.
YTHDC2 stands out due to its RNA helicase domain and is pivotal in meiotic progression. It enhances translation efficiency while simultaneously promoting mRNA degradation, demonstrating a dual role in maintaining transcriptome balance.
Interestingly, recent studies suggest that YTH family members can form phase-separated compartments—structures such as P bodies and stress granules—through multivalent interactions with m6A-rich transcripts. This property enables cells to dynamically regulate RNA fate during stress, differentiation, or disease.
Together, these readers ensure that m6A-modified RNAs are handled with precision, dictating whether a message is translated, silenced, or destroyed—a vital layer of post-transcriptional gene control with profound implications for cancer biology and beyond.
Structure Equals Function: How YTH Domains Recognize m6A
The functional diversity of YTH family proteins in RNA regulation stems largely from their ability to specifically recognize m6A-modified RNA through a highly conserved YTH domain. Structural studies have revealed that this domain is not merely a passive binder, but a precision-engineered molecular reader, uniquely suited to identify methylated adenosines among a sea of RNA bases.
Each YTH domain forms a mixed α-helix and β-sheet fold, with a distinctive aromatic cage at its core. This cage typically consists of conserved tryptophan and leucine residues, which stabilize the m6A modification through π–π stacking and hydrophobic interactions. For example, in YTHDC1, the residues W377, W428, and L439 form a binding pocket that fits the m6A mark like a glove. Mutation of these residues abolishes binding, highlighting their essential role.
Beyond the hydrophobic interactions, hydrogen bonding further reinforces the specificity. Residues such as N363, S378, and N367 in YTHDC1 form hydrogen bonds with the N1, N3, and N6 atoms of the adenine ring. Interestingly, substitution of N367 with an aspartic acid (D367) significantly reduces m6A affinity, underscoring the sensitivity of the binding site to atomic-level changes.
Electrostatic surface analysis also reveals that the YTH domain contains positively charged patches, rich in arginine and lysine, which facilitate RNA backbone binding. These basic residues (e.g., R411, K416, R441, R527 in YTHDF2) contribute to the domain’s high affinity for RNA but are not directly involved in m6A recognition.
Among YTH proteins, YTHDC1 is unique in showing sequence selectivity for nucleotides adjacent to m6A, particularly favoring a guanine at the -1 position. This selectivity arises from hydrogen bonding and van der Waals interactions with unique residues like L380 and M438—features absent in other YTH proteins like YTHDF1 or YTHDF2.
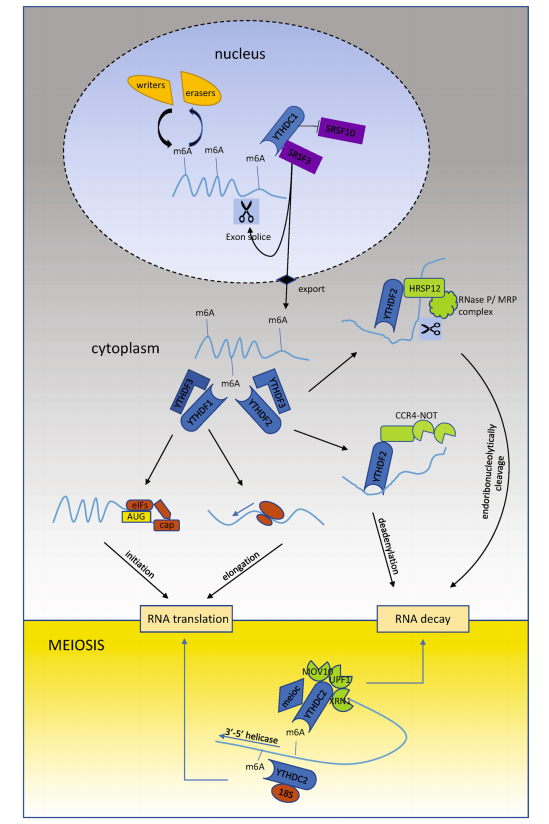
Fig. 1 Model of YTH family proteins modulating m6A-containing RNAs.
These detailed structural insights provide not only a molecular explanation for m6A reading but also a promising foundation for drug design. The well-defined m6A binding pocket of YTH domains makes them attractive targets for small-molecule inhibitors, particularly in cancer therapy where disrupting m6A recognition could have therapeutic benefits.
Cancer Connections: How YTH Proteins Drive or Suppress Tumors
The dynamic influence of m6A modifications extends far beyond normal gene regulation—it plays a pivotal role in cancer development, progression, and therapy resistance. As primary readers of m6A, YTH domain-containing proteins serve as key regulators in tumor biology, acting either as oncogenes or tumor suppressors, depending on cellular context and cancer type.
One of the most widely studied is YTHDF1, which enhances the translation of oncogenic transcripts. In colorectal cancer, YTHDF1 promotes Wnt signaling by facilitating the translation of TCF4, a key transcription factor in the β-catenin pathway. Similarly, in gastric cancer, it boosts the translation of FZD7, another Wnt pathway component. In non-small cell lung cancer (NSCLC), YTHDF1 drives tumor proliferation by targeting CDK2, CDK4, and YAP, leading to enhanced cell cycle progression and metastasis.
YTHDF1’s role extends into immune evasion as well. It promotes the translation of lysosomal proteases in dendritic cells, accelerating neoantigen degradation and blunting anti-tumor immunity, thereby limiting immunotherapy efficacy. Despite its generally oncogenic profile, YTHDF1 has shown a tumor-suppressive role in ocular melanoma, where it enhances translation of the tumor suppressor HINT2.
In contrast, YTHDF2 is often associated with mRNA degradation and displays both oncogenic and tumor-suppressive behavior. In hepatocellular carcinoma (HCC), YTHDF2 destabilizes IL11 and SERPINE2 mRNAs, curbing inflammation and tumor vasculature reprogramming—thus functioning as a tumor suppressor. However, in other contexts like glioblastoma, YTHDF2 promotes oncogenesis by degrading transcripts like LXRA and stabilizing MYC and VEGFA, enhancing proliferation and invasion.
YTHDF3, though less studied, appears to coordinate YTHDF1 and YTHDF2 activity. It plays a critical role in breast cancer brain metastasis, enhancing translation of EGFR, ST6GALNAC5, and GJA1. Additionally, YTHDF3 facilitates epithelial–mesenchymal transition (EMT) through Snail translation and influences YAP signaling by directing mRNA to either translation or decay pathways.
The duality of YTH proteins—as both oncogenic drivers and suppressors—highlights their context-dependent roles. Their emerging significance in multiple cancer types not only deepens our understanding of epitranscriptomic regulation but also positions them as promising targets for precision oncology.
Future Outlook: Targeting YTH Proteins for Cancer Therapy
As the role of m6A modifications in gene regulation and cancer becomes increasingly clear, the spotlight is turning to YTH family proteins as potential therapeutic targets. While many early studies focused on m6A “writers” (e.g., METTL3) and “erasers” (e.g., FTO), emerging evidence suggests that readers—especially the YTH domain-containing proteins—may offer more precise intervention points for controlling oncogenic gene expression.
The rationale is compelling: YTH proteins act downstream of the m6A pathway and directly determine RNA fate, influencing whether a transcript is stabilized, translated, exported, or degraded. This central position makes them ideal candidates for targeted drug development, particularly in cancer types where m6A regulation is hijacked to support tumor growth, metastasis, or therapy resistance.
Recent structural studies have paved the way for structure-guided drug design. The aromatic cage in the YTH domain, which precisely recognizes m6A-modified bases, offers a defined and druggable binding pocket. In a recent breakthrough, computational screening and crystallography were used to identify small-molecule fragments—notably N-methyl amide analogs—that could competitively bind the m6A recognition site in YTHDC1, thereby blocking its interaction with methylated RNA.
Although these findings are preliminary, they open the door to a new class of epitranscriptomic drugs: molecules that selectively inhibit m6A readers without disrupting global methylation patterns. This level of specificity could reduce off-target effects and improve therapeutic windows in cancers driven by YTHDF or YTHDC dysregulation.
Another future direction lies in unraveling the redundancy and plasticity among YTH proteins. Some models suggest that YTHDF1, YTHDF2, and YTHDF3 may have overlapping or even compensatory functions, raising important questions about how best to inhibit them therapeutically. Could a single inhibitor block all YTHDFs? Or would combination strategies be more effective?
Finally, there is strong potential for integrating YTH-targeted therapies with existing cancer treatments, such as immunotherapies or chemotherapeutics, especially given the role of YTHDF1 in suppressing anti-tumor immune responses.
As the field of epitranscriptomic oncology matures, targeting YTH readers may prove to be a transformative strategy for treating cancers that have long resisted conventional therapies.
References
Desrosiers, R., Friderici, K., & Rottman, F. (1974). Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proceedings of the National Academy of Sciences, 71(10), 3971–3975.
https://doi.org/10.1073/pnas.71.10.3971
Dominissini, D., Moshitch-Moshkovitz, S., Schwartz, S., et al. (2012). Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature, 485(7397), 201–206.
https://doi.org/10.1038/nature11112
Meyer, K. D., Saletore, Y., Zumbo, P., et al. (2012). Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell, 149(7), 1635–1646.
https://doi.org/10.1016/j.cell.2012.05.003
Wang, X., Zhao, B. S., Roundtree, I. A., et al. (2015). N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell, 161(6), 1388–1399.
https://doi.org/10.1016/j.cell.2015.05.014
Du, H., Zhao, Y., He, J., et al. (2016). YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4–NOT deadenylase complex. Nature Communications, 7, 12626.
https://doi.org/10.1038/ncomms12626
Xiao, W., Adhikari, S., Dahal, U., et al. (2016). Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Molecular Cell, 61(4), 507–519.
https://doi.org/10.1016/j.molcel.2016.01.012
Xu, C., Wang, X., Liu, K., et al. (2014). Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nature Chemical Biology, 10(11), 927–929.
https://doi.org/10.1038/nchembio.1654
Zhu, T., Roundtree, I. A., Wang, P., et al. (2014). Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Research, 24(12), 1493–1496.
https://doi.org/10.1038/cr.2014.152
Li, F., Zhao, D., Wu, J., & Shi, Y. (2014). Structure of the YTH domain of human YTHDF2 in complex with an m6A mononucleotide reveals an aromatic cage for m6A recognition. Cell Research, 24(12), 1490–1492.
https://doi.org/10.1038/cr.2014.153
Shi, R., Ying, S., Li, Y., Zhu, L., Wang, X., & Jin, H. (2021). Linking the YTH domain to cancer: The importance of YTH family proteins in epigenetics. Cell Death & Disease, 12, 346.
https://doi.org/10.1038/s41419-021-03625-8
Chang, G., Shi, L., Ye, Y., et al. (2020). YTHDF3 induces the translation of m6A-enriched gene transcripts to promote breast cancer brain metastasis. Cancer Cell, 38(6), 857–871.e7.
https://doi.org/10.1016/j.ccell.2020.10.004
Moroz-Omori, E. V., Huang, D., Bedi, R. K., et al. (2021). METTL3 inhibitors for epitranscriptomic modulation of cellular processes. Nature Communications, 12, 1136.
https://doi.org/10.1038/s41467-021-21410-4